Abstract
Health-related quality of life (HRQL) outcomes evaluation is becoming an important component of clinical trials of new pharmaceuticals and medical devices. HRQL research provides patients, providers, and decision makers with important information on the impact of disease and treatment on physical, psychological, and social functioning and well-being. These outcomes are also useful to the pharmaceutical and device industries as they attempt to understand and communicate product value to physicians, patients, health insurers and others. HRQL labeling and promotional claims in the US are likely to increase over the next few years. The evidentiary requirements to make such a claim should be based on accepted scientific standards of HRQL evaluation and consistent with the regulatory requirements for clinical efficacy. This report outlines the scientific practices that should be considered in the evaluation of evidence for an HRQL claim, including the selection of appropriate domains, evidence to support the reliability and validity of HRQL measurement, considerations in research design and statistical analyses, and the issue of clinical significance. Representatives from the pharmaceutical and device industries, regulatory agencies, and the HRQL scientific community should work together to make certain the use of HRQL in labeling and promotion are based on sound scientific evidence, and that these messages are clearly and accurately reported to the consumers.
Similar content being viewed by others
References
FDA Modernization Act, 1997. Available at: http:// www.fda.gov/cder/guidance/105-115. Accessed 7 January 1999.
Bergner M. Quality of life, health status, and clinical research. Med Care 1989; 27(Suppl): S148–S156.
Leidy NK. Using functional status to assess treatment outcomes. Chest 1994; 106(6): 1645–1646.
Cella DF, Tulsky DS. Measuring quality of life today: Methodological aspects. Oncology 1990; 5: 29–38.
Levine S, Croog S. What constitutes quality of life? A conceptualization of the dimensions of life quality in heal-thy populations and patients with cardiovascular disease. In: Wenger N, Mattson M, Furberg C, Elinson J (eds), Assessment of Quality of Life in Clinical Trials of Car-diovascular Therapies. Greenwich, CT: La Jacq, 1984: 46-58.
Patrick DL, Erickson P. Health Status and Health Policy: Allocating Resources to Health Care. New York: Oxford University Press, 1993.
Barofsky I, Sugarbaker PH. Cancer. In: Spilker B (ed), Quality of Life Assessments in Clinical Trials. New York: Raven Press, 1990: 419–440.
Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA 1994; 272: 619–626.
Schipper H, Clinch JJ, Olweny CL. Quality of life studies: Definitions and conceptual issues. In: Spilker B (ed), 898 Quality of Life and Pharmacoeconomics in Clinical Trials, 2nd edn. Philadelphia: Lippincott-Raven, 1996: 11–24.
Leplege A, Hunt S. The problem of quality of life in med-icine. JAMA 1997; 278: 47–50.
Joyce CRB, O'Boyle CA, McGee H(eds), Individual Quality of Life: Approaches to Conceptualisation and Assessment. Amsterdam: Harwood Academic Publishers, 1999.
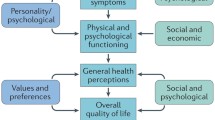
Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. JAMA 1995; 273: 59–65.
Ware JE. Methodological considerations in the selection of health status assessment procedures. In: Wenger N, Matt-son M, Furberg C, Elinson J (eds), Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. Greenwich, CT: La Jacq, 1984: 87–111.
Leidy NK, Revicki DA, Geneste B. Recommendations for evaluating the validity of quality of life claims for labeling and promotion. Value in Health 1999; 2: 113–127.
Guyatt GH, Feeny D, Patrick DL. Issues in quality of life measurement in clinical trials. Control Clin Trials 1991; 12: 81S–90S.
Shumaker SA, Berzon R. The International Assessment of Health-Related Quality of Life: Theory, Translation, Measurement, and Analysis. Oxford: Rapid Communica-tions, 1995.
Revicki DA. Health care technology assessment and health-related quality of life. In: Banta D, Luce BR (eds), Health Care Technology and its Assessment: An International Perspective. New York: Oxford University Press, 1993: 114–131.
Gantz PA. Quality of life and the patient with cancer: In-dividual and policy implications. Cancer 1994; 74: 1445–1452.
Revicki DA, Ehreth JL. Health-related quality of life as-sessment and planning for the pharmaceutical industry. Clin Ther 1997; 19: 1101–1115.
Spilker B (ed), Quality of Life and Pharmacoeconomics in Clinical Trials, 2nd edn. Philadelphia: Lippincott-Raven, 1996.
Staquet M, Hays R, Fayers P (eds), Quality of Life As-sessment in Clinical Trials. New York: Oxford University Press, 1998.
McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires, 2nd edn. New York: Oxford University Press, 1996.
Berzon RA, Donnelly MA, Simpson RL, Simeon GP, Til-son HH. Quality of life bibliography and indexes. Qual Life Res 1994; 6: 547–568.
McHorney CA. Generic health measurement: Past accom-plishments and a measurement paradigm for the 21st century. Ann Intern Med 1997; 127: 743–750.
Nunnally JC, Bernstein IH. Psychometric Theory, 3rd edn. New York: McGraw-Hill, 1994.
American Psychological Association. Standard for Educa-tional and Psychological Tests. Washington, DC: American Psychological Association, 1985.
Guyatt GH, Kirshner B, Jaeschke R. Measuring health status: What are the necessary measurement properties? J Clin Epidemiol 1992; 45: 1341-1345.
Lohr KN, Aaronson NK, Alonso J, et al. Evaluating quality of life and health status instruments: Development of scientific review criteria. Clin Ther 1996; 18: 979–992.
Hays R, Anderson R, Revicki DA. Assessing reliability and validity of measurement in clinical trials. In: Staquet M, Hays R, Fayers P (eds), Quality of Life Assessment in Clinical Trials. New York: Oxford University Press, 1998: 169–182.
Cronbach LJ, Meehl PE. Construct validity in psycholog-ical tests. Psychol Bull 1955; 52: 281-302.
Stewart AL, Hays RD, Ware JE. Methods of validating MOS health measures. In: Stewart AL, Ware JE (eds), Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham: Duke University Press, 1992: 309–324.
Hadorn DC, Hays RD. Multitrait-multimethod analysis of health-related quality of life preferences. Med Care 1991; 29: 829–840.
Hays R, Hadorn D. Responsiveness to change: An aspect of validity, not a separate dimension. Qual Life Res 1992; 1: 73–75.
Guyatt GH, Walter S, Norman G. Measuring change over time: Assessing the usefulness of evaluative instruments. J Chronic Dis 1987; 40: 171–178.
Kazdin AE. The meaning and measurement of clinical significance. J Consult Clin Psychol 1999; 67: 332-339.
Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16: 139–144.
Lydick E, Epstein RS. Interpretation of quality of life changes. Qual Life Res 1993; 2: 221–226.
Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press, 1977.
Kazis LE, Anderson JJ, Meenan RF. Effect sizes for in-terpreting changes in health status. Med Care 1989; 27(suppl): S178-S189.
Jaeschke R, Singer J, Guyatt GH. Measurement of health status: Ascertaining the minimal clinically important dif-ference. Control Clin Trials 1989; 10: 407–415.
Juniper EF, Guyatt GH, Willan A, et al. Determining a minimal important change in a disease-speci®c quality of life questionnaire. J Clin Epidemiol 1994; 47: 81–87.
Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual.Boston: The Health Institute, New England Medical Center, 199
King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res 1996; 5: 555–567.
Norman GR. Issues in the use of change scores in ran-domized trials. J Clin Epidemiol 1989; 42(11): 1097–1105.
Norman GR, Stratford P, Regehr G. Methodological problems in the retrospective computation of responsive-ness to change: The lesson of Cronbach. J Clin Epidemiol 1997; 50(8): 869–879.
Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient's view of change as a clinical outcome measure. JAMA 1999; 282: 1157–1162.
Wyrwich KM, Tierney WM, Wolinsky FD. Further evi-dence supporting an SEM-based criterion for identifying 899 meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999; 57: 861-873.
Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care 1999; 37: 469-478.
Testa MA. Interpretation of quality-of-life outcomes: Is-sues that afect magnitude and meaning. Med Care 2000; 38(suppl 9): 166-174.
Revicki DA. Instrument Selection and Validation: How Much is Enough? Regulatory Issues in Health-Related Quality of Life Assessment. Washington, DC: PhRMA/ FDA Interactive Workshop, March 1999.
Meinhart C, Tonascia S. Clinical Trials Design, Conduct and Analysis. New York: Oxford University Press, 1986.
Bernhard J, Cella DF, Coates AS, et al. Missing quality of life data in clinical trials: Serious problems and challenges. Stat Med 1998; 17: 517-532.
Bonomi A, Legro M, Leidy NK, et al. Maximizing quality in quality of life outcomes research: A multi-faceted ap-proach for clinical trials. Paper presented at the Annual Conference of the International Society for Quality of Life Research, Baltimore MD, November 1998.
Little RJA. Modeling the drop-out mechanism in repeated-measures studies. J Am Stat Assoc 1995; 90: 1112-1121.
Jennrich R, Schluchter M. Unbalanced repeated-measures models with structured covariance matrices. Biometrics 1986; 42: 805-820.
Laird NM, Ware JH. Random-effects models for longitu-dinal data. Biometrics 1982; 38: 963-974.
Fairclough DL, Peterson HF, Cella D, Bonomi P. Com-parison of several model-based methods for analyzing in-complete quality of life data in cancer clinical trials. Stat Med 1998; 17: 781-796.
Hedeker D, Gibbons RD. Application of random-efects pattern-mixture models for missing data in longitudinal studies. Psych Methods 1997; 2: 64-78.
Revicki DA, Gold K, Buckman D, Chan K, Kallich JD, Woolley M. Imputing physical function scores missing owing to mortality: Results of a simulation comparing multiple techniques. Med Care (in press).
Fairclough DL. Summary measures and statistics for comparison of quality of life in a clinical trial of cancer therapy. Stat Med 1997; 16: 1197-1209.
Zhang J, Quan H, Ng J, Stepanavage ME. Some statistical methods for multiple endpoints in clinical trials. Control Clin Trials 1997; 18: 204-221.
O'Brien PC. Procedures for comparing samples with mul-tiple endpoints. Biometrics 1984; 40: 511-521.
Revicki DA, Moyle G, Stellbrink HJ, Barker C. Quality of life outcomes of combination zalcitabine-zidovudine, squ-inavir-zidovudine, and saquinavir-zalcitabine-zidovudine therapy for HIV-infected adults with CD4 counts between 50 and 350 cells per cubic millimeter. AIDS 1999; 13: 851-858.
Wu AW, Gray SM, Brookmeyer R. Application of random efects models and other methods to the analysis of multi-dimensional quality of life data in AIDS clinical trials. Med Care 1999; 37: 249-258.
Burke L. Regulatory Issues in the Use of Patient Reported Outcomes in Drug Labeling and Advertising. Presented at the Health-Related Quality of Life Workshop, Center for Drug Evaluation and Research, Food and Drug Adminis-tration, Rockville, MD, September 28, 2000.
Bukstein DA. Incorporating quality of life data into man-aged care formulary decisions: A case study with salme-terol. Am J Man Care 1997; 3: 1701-1706.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Revicki, D., Osoba, D., Fairclough, D. et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res 9, 887–900 (2000). https://doi.org/10.1023/A:1008996223999
Issue Date:
DOI: https://doi.org/10.1023/A:1008996223999