Abstract
Purpose. The medium chain fatty acid sodium caprate (C10) is approved as an absorption enhancer but its mechanism of action has not been studied in humans. The aim of this study was to investigate the mechanism of action of C10 in human subjects after rectal administration.
Methods. Twelve healthy human subjects were randomised to receive ampicillin suppositories with (AM-C10) or without (AM) C10. Serum and urine samples were collected and analysed for ampicillin by HPLC. Rectal biopsies were taken before and 25 min (approximate time of maximum serum concentration, Cmax, for ampicillin) and 185 min (during the final part of the elimination phase) after rectal administration of the suppositories. The osmolality of the rectal fluid was also measured.
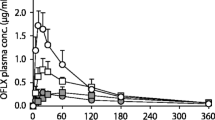
Results. AM-C10 administration increased Cmax, area under the serum concentration-time curve (AUC) and urinary recovery of ampicillin 2.6-, 2.3- and 1.8-fold, respectively, compared to AM. Histological examination of the biopsies showed that AM-C10 exposure resulted in reversible mucosal damage that occurred at the same time as the Cmax for ampicillin while AM prolonged mucosal damage. A reversible increase in rectal fluid osmolality was observed with both treatments.
Conclusions. AM-ClO-enhanced absorption of ampicillin coincides with non-specific damage to the rectal mucosa. C10 itself as well as the suppository base and the hyperosmolality of the rectal fluid contributed to this effect. However, the histological damage was reversible with AM-C10, suggesting that C10 also has a protective effect on the rectal mucosa.
Similar content being viewed by others
REFERENCES
T. Nishihata, J. H. Rytting, and T. Higuchi. J. Pharm. Sci. 70:71–75 (1981).
E. J. van Hoogdalem, C. Vermeij-Kerrs, A. G. de Boer, and D. D. Breimer. J. Pharm. Sci. 79:866–870 (1990).
K. Nakanishi, M. Masada, and T. Nadai. Chem. Pharm. Bull. 31:4161–4166 (1983).
E. S. Swenson, W. B. Milisen, and W. Curatolo. Pharm. Res. 11:1132–1142 (1994).
E. S. Swenson and W. J. Curatolo. Adv. Drug Delivery Rev. 8:39–92 (1992).
B. J. Aungst, H. Saitoh, D. L. Burcham, S.-M. Huang, S. A. Mousa, and M. A. Hussain. J. Controll. Release 41:19–31 (1996).
E. K. Anderberg, T. Lindmark, and P. Artursson. Pharm. Res. 10:857–864 (1993).
T. Sawada, T. Ogawa, M. Tomita, M. Hayashi, and S. Awazu. Pharm. Res. 8:1365–1371 (1991).
K. Takahashi, T. Murakami, R. Yumoto, T. Hattori, Y. Higashi, and N. Yata. Pharm. Res. 11:1401–1404 (1994).
Swedis. Data obtained from Swedis data base; Medical Products Agency, Uppsala, Sweden.
J. Lal, J. K. Paliwal, P. K. Grover, and R. C. Gupta. J. Chromatogr. B 655:142–146 (1994).
I. Nordgaard-Andersen, M. Rye Clausen, and P. Brobech Mortensen. J. Parenter. Enteral Nutr. 34:324–331 (1993).
M. Mishima, A. Nagatomi, T. Yamakita, Y. Miura, and O. Tsuzuki. Biol. Pharm. Bull. 18:566–570 (1995).
E. J. van Hoogdalem, A. G. de Boer, and D. D. Breimer. Pharm. Weekbl. (Sci.) 10:76–79 (1988).
H. Yaginuma, Y. Isoda, Y. Wada, S. Itoh, M. Yamazaki, A. Kamada, H. Shimazu, and I. Makita. Chem. Pharm. Bull. 30:1073–1076 (1982).
K. I. Nishimura, Y. Nozaki, A. Yoshimi, S. Nakamura, M. Kitagawa, N. Kakeya, and K. Kitao. Chem. Pharm. Bull. 33:282–291 (1985).
M. Tomita, M. Shiga, M. Hayashi, and S. Awazu. Pharm. Res. 5:341–346 (1988).
J. D. Söderholm, L. Hedman, and G. Olaison. Tight junctional permeability in human ileal mucosa—modulation with sodium caprate and cytochalasin B. Gut. 37(suppl 2):A39 (1995).
E. K. Anderberg and P. Artursson. J. Pharm. Sci. 82:392–398 (1993).
E. K. Anderberg, C. Nyström, and P. Artursson. J. Pharm. Sci. 81:879–887 (1992).
T. Lindmark, T. Nikkilä, and P. Artursson. J. Pharmacol. Exp. Ther. 275:958–964 (1995).
M. Tomita, M. Hayashi, and S. Awazu. J. Pharmacol. Exp. Ther. 272:739–743 (1995).
A. G. de Boer, E. J. van Hoogdalem, and D. D. Breimer. Adv. Drug Delivery Rev. 8:237–251 (1992).
Dr. C. Graffner. Astra Läkemedel AB, Sweden. (personal communication).
K. Takahashi, T. Murakami, A. Kamata, R. Yumoto, Y. Higashi, and N. Yata. Pharm. Res. 11:388–392 (1994).
L. Lohikangas, M. Wilen, M. Einarsson, and P. Artursson. Eur. J. Pharm. Sci. 1:307–312 (1994).
C. De Muynck, C. Cuvelier, D. Van Steenkiste, L. Bonnarens, and J. P. Remon. Pharm. Res. 8:945–950 (1991).
N. Yata, W. M. Wu, R. Yamajo, T. Murakami, Y. Higashi, and T. Higuchi. J. Pharm. Sci. 74:1058–1061 (1985).
Y. Kimura, T. Lindmark, and P. Artursson. Regulation of immediate and long term effects of the absorption enhancer sodium caprate in human intestinal epithelial (Caco-2) cells. Proc. 23rd Int. Symp. Control. Rel. Bioact. Mater. 423–424 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindmark, T., Söderholm, J.D., Olaison, G. et al. Mechanism of Absorption Enhancement in Humans After Rectal Administration of Ampicillin in Suppositories Containing Sodium Caprate. Pharm Res 14, 930–935 (1997). https://doi.org/10.1023/A:1012112219578
Issue Date:
DOI: https://doi.org/10.1023/A:1012112219578