Abstract
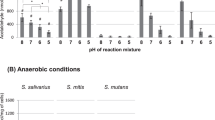
CARCINOGENIC nitrosamines are formed by chemical reaction between nitrous acid and secondary and some tertiary amines in the stomachs of rodents1–3. Nitrosamines could be formed similarly in the human stomach and so present a carcinogenic hazard to man. The nitrosation reaction is catalysed by some anions4 particularly thiocyanate5,6. Thiocyanate is secreted in saliva which in non-smokers normally contains about 50 mg l−1 (approximately 1 mM) but in smokers contains three to four times this concentration7. In the absence of catalyst the nitrosation of N-methylaniline and some other secondary amines is maximal at pH 3, but in the presence of thiocyanate the reaction proceeds much more rapidly in acid conditions, such as in gastric juice, between pH 1 and 2 (Fig. 1). At pH 1.5, 1.0 mM thiocyanate increases the rate of reaction about 550 times. Under these acid conditions below pH 2 the nitrosation reaction rate is proportional to the concentrations of (1), nitrite; (2), thiocyanate; and (3), amine8. At pH 3.5 and greater thiocyanate has less catalytic action and the predominant reaction is proportional to the square of the nitrite concentration, but is slow.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sander, J., and Bürkle, G., Z. Krebsforsch, 75, 301 (1969).
Mirvish, S. S., Greenblatt, M., and Kommineni, U. R. C., J. natn. Cancer Inst., 48, 1311 (1972).
Lijinsky, W., Taylor, H. W., Snyder, C., and Nettesheim, P., Nature, 244, 176 (1973).
Ridd, J. H., Q. Rev., 15, 418 (1961).
Boyland, E., Nice, E., and Williams, K., Food cosmet. Toxicol., 9, 639 (1971).
Fan, T-Y., and Tannenbaum, S. R., J. agric. Fd Chem., 21, 14 (1973).
Densen, P. M., Davidow, B., Bass, H. E., and Jones, E. W., Archs envir. Hlth, 14, 865 (1967).
Boyland, E., and Walker, S. A., Nitroso Compounds Analysis and Formation (International Agency for Research on Cancer, Lyon, in the press).
Schönbein, Jber. chem. p. 98 (1862).
Ville, J., and Mestrezat, W., C. r. Séanc. Soc. Biol., 63, 231 (1907).
Barylko-Pikielna, N., and Panghorn, R. M., Archs envir. Hlth, 17, 739 (1968).
Schievelbein, H., Werle, E., Schulz, E. K., and Banmeister, R., Arch. Pharmak exp. Path., 262, 358 (1969).
Hoffman, D., Rathcamp, G., and Liu, Y. Y., Nitroso Compounds Analysis and Formation (International Agency for Research on Cancer, Lyon, in the press).
Boyland, E., Roe, F. J. C., and Gorrod, J. W., Nature, 202, 1126 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BOYLAND, E., WALKER, S. Effect of thiocyanate on nitrosation of amines. Nature 248, 601–602 (1974). https://doi.org/10.1038/248601a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/248601a0
This article is cited by
-
A review: dietary and endogenously formed N-nitroso compounds and risk of childhood brain tumors
Cancer Causes & Control (2005)
-
Chronic atrophic gastritis and risk of n-nitroso compounds carcinogenesis
Langenbecks Archiv f�r Chirurgie (1988)
-
Formation in aqueous solution of N-nitroso curzate and the catalytic effect of some anions
Bulletin of Environmental Contamination and Toxicology (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.