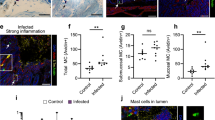
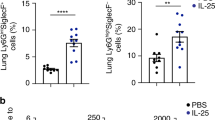
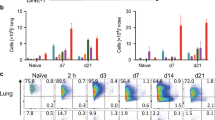
Abstract
ALTHOUGH mast cells have been implicated in a variety of inflammatory conditions including immediate hypersensitivity and interstitial cystitis,1,2 their physiological role in the body is unknown. We investigated the role of mast cells in host defence against bacterial infections using a well characterized mast-cell-deficiency mouse model3,4. We report here that mast cells, which are selectively located at portals of bacterial entry, are important to host defence. Mast-cell-deficient WBB6F1-W/Wv mice (W/Wv) were up to 20-fold less efficient in clearing enterobacteria than control WBB6F1+/+ (+/+) mice or mast-cell-reconstituted W/Wv (W/Wv + MC) mice. With higher bacterial inocula, only W/Wv mice died (80%). The limited bacterial clearance in W/Wv mice directly correlated with impaired neutrophil influx. The mast-cell chemoattractant TNF-α was implicated in the neutrophil response because TNF-α was locally released only in +/+ and W/Wv + MC mice, TNF-α-specific antibodies blocked over 70% of the neutrophil influx, and purified mast cells released TNF-α upon incubation with bacteria. Additionally, the type-1 fimbrial subunit, FimH, was the necessary enterobacterial component for mast-cell activation and neutrophil influx because an isogenic FimH– mutant evoked a limited neutrophil response in +/+ mice compared to wild-type bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Galli, S. J. New Engl. J. Med. 328, 257–263 (1993).
Malone, D. G. & Metcalfe, D. D. Ann. Allergy 61, 27–30 (1988).
Zhang, Y., Ramos, B. F. & Jakschik, B. A. Science 258, 1957–1959 (1992).
Kitamura, Y., Go, S. & Hatanaka, K. Blood 52, 447–452 (1978).
Bienenstock, J., Befus, A. D., Pearce, F., Denburg, J. & Goodacre, R. J. Allergy clin. Immun. 70, 407–412 (1982).
Malaviya, R. et al. J. Immun. 152, 1907–1914 (1994).
Malaviya, R., Ross, E. A., Jakschik, B. A. & Abraham, S. N. J. clin. Invest. 93, 1645–1653 (1994).
Malaviya, R., Twesten, N. J., Ross, E. A., Abraham, S. N. & Pfeifer, J. D. J. Immun. 156, 1490–1496 (1996).
Gerlach, C. F., Clegg, S. & Allen, B. J. Bact. 171, 1262–1270 (1989).
Abraham, S. N., Sun, D., Dale, J. B. & Beachy, E. H. Nature 336, 682–684 (1988).
Madison, B., Ofek, I., Clegg, S. & Abraham, S. N. Infect. Immun. 62, 843–848 (1994).
Gordon, J. R. & Galli, S. J. J. exp. Med. 174, 103–117 (1991).
Wershil, B. K., Wang, Z.-H., Gordon, J. R. & Galli, S. J. J. clin. Invest. 87, 446–453 (1991).
Vassalli, P. A. Rev. Immun. 10, 411–452 (1992).
Natanson, C., Hoffman, W. D., Suffredini, A. F., Eichacker, P. Q. & Danner, R. L. A. Intern. Med. 120, 771–783 (1994).
Arnold, J. W. et al. Microbiol. Pathogen. 14, 217–227 (1993).
Echtenacher, B., Falk, W., Mannel, D. N. & Krammer, P. H. J. Immun. 145, 3762–3766 (1990).
Kitamura, Y. et al. J. exp. Med. 150, 482–490 (1979).
Nakano, T. et al. J. exp. Med. 162, 1025–1043 (1985).
Suzuki, N. et al. Am. J. Resp. cell Molec. Biol. 9, 475–483 (1993).
Ofek, I. et al. Infect. Immun. 61, 4208–4216 (1993).
Esposito, A. L., Quinn, L. A., Lucey, E. C. & Snider, G. L. Am. Rev. respir. Dis. 135, 676–688 (1987).
Bradley, P. P., Priebat, D. A., Christensen, R. D. & Rothstein, G. J. Invest. Dermatol. 78, 206–209 (1982).
Sheehan, K. C. F., Ruddle, N. H. & Schreiber, R. D. J. Immun. 142, 3884–3893 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Malaviya, R., Ikeda, T., Ross, E. et al. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381, 77–80 (1996). https://doi.org/10.1038/381077a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/381077a0
This article is cited by
-
A two-step activation mechanism enables mast cells to differentiate their response between extracellular and invasive enterobacterial infection
Nature Communications (2024)
-
Immunomodulation therapy offers new molecular strategies to treat UTI
Nature Reviews Urology (2022)
-
Neutrophil breaching of the blood vessel pericyte layer during diapedesis requires mast cell-derived IL-17A
Nature Communications (2022)
-
A bibliometric analysis of top-cited journal articles in interstitial cystitis and bladder pain syndrome
International Urogynecology Journal (2022)
-
IgE-activated mast cells enhance TLR4-mediated antigen-specific CD4+ T cell responses
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.