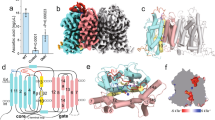
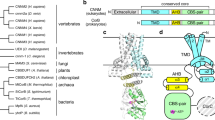
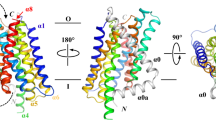
Abstract
Metal ions are essential cofactors for a wealth of biological processes, including oxidative phosphorylation, gene regulation and free-radical homeostasis. Failure to maintain appropriate levels of metal ions in humans is a feature of hereditary haemochromatosis1, disorders of metal-ion deficiency, and certain neurodegenerative diseases2. Despite their pivotal physiological roles, however, there is no molecular information on how metal ions are actively absorbed by mammalian cells. We have now identified a new metal-ion transporter in the rat, DCT1, which has an unusually broad substrate range that includes Fe2+, Zn2+, Mn2+, Co2+, Cd2+, Cu2+, Ni2+ and Pb2+. DCT1 mediates active transport that is proton-coupled and depends on the cell membrane potential. It is a 561-amino-acid protein with 12 putative membrane-spanning domains and is ubiquitously expressed, most notably in the proximal duodenum. DCT1 is upregulated by dietary iron deficiency, and may represent a key mediator of intestinal iron absorption. DCT1 is a member of the ‘natural-resistance-associated macrophage protein’ (Nramp) family3,4,5 and thus its properties provide insight into how these proteins confer resistance to pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feder, J. N. et al. Anovel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genet. 13, 399–408 (1996).
Hirsch, E. C. Biochemistry of Parkinson's disease with special reference to the dopaminergic systems. Mol. Neurol. 94, 135–142 (1997).
Gruenheid, S., Cellier, M., Vidal, S. & Gros, P. Identification and characterization of a second mouse Nramp gene. Genomics 25, 514–525 (1995).
Vidal, S., Malo, D., Vogan, K., Skamene, E. & Gros, P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73, 469–485 (1993).
Vidal, S., Gros, P. & Skamene, E. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J. Leuk. Biol. 58, 382–390 (1995).
Conrad, M. E., Umbreit, J. N., Moore, E. G. & Heiman, D. Mobilferrin is an intermediate in iron transport between transferrin and hemoglobin in K562 cells. J. Clin. Invest. 98, 1449–1454 (1996).
Raja, K. B., Simpson, R. J. & Peters, T. J. Investigation of a role for reduction in ferric iron uptake by mouse duodenum. Biochim. Biophys. Acta 1135, 141–146 (1992).
Jordan, I. & Kaplan, J. The mammalian transferrin-independent iron transport system may involve a surface ferrireductase activity. Biochem. J. 302, 875–879 (1994).
Han, O., Failla, M. L., Hill, A. D., Morris, E. R. & Smith, J. C. Reduction of Fe(III) is required for uptake of nonheme iron by Caco-2 cells. J. Nutr. 125, 1291–1299 (1995).
Dorey, C. et al. Iron speciation at physiological pH in media containing ascorbate and oxyten. Br. J. Nutr. 70, 157–169 (1993).
Eide, D. J. Molecular biology of iron and zinc uptake in eukaryotes. Curr. Opin. Cell Biol. (in the press).
Palmiter, R. D., Cole, T. B., Quaife, C. J. & Findley, S. D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA 93, 14934–14939 (1996).
Bull, P. & Cox, D. W. Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet. 10, 246–252 (1994).
Nussberger, S. et al. Symmetry of H+ binding to the intra- and extracellular side of the H+-coupled oligopeptide cotransporter PepT1. J. Biol. Chem. 272, 7777–7785 (1997).
Mackenzie, B. et al. Mechanisms of the human intestinal H+-coupled oligopeptide transporter hPEPT1. J. Biol. Chem. 271, 5430–5437 (1996).
Boorer, K. J., Loo, D. D. F. & Wright, E. M. Steady-state and presteady-state kinetics of the H+/hexose cotransporter (STP1) from Arabidopsis thaliana expressed in Xenopus oocytes. J. Biol. Chem. 269, 20417–20424 (1994).
Mager, S. et al. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron 10, 177–188 (1993).
McEwan, G. T. A., Daniel, H., Fett, C., Burgess, M. N. & Lucas, M. L. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc. R. Soc. Lond. 234, 219–237 (1988).
Mackenzie, B., Loo, D. D. F. & Wright, E. M. Coupling stoichiometry for the Na+/glucose cotransporter SGLT1.(manuscript in preparation).
Boorer, K. J. et al. Kinetics and specificity of a H+/amino acid transporter from Arabidopsis thaliana J. Biol. Chem. 271, 2213–2220 (1996).
Mackenzie, B., Loo, D. D. F., Panayotova-Heiermann, M. & Wright, E. M. Biophysical characteristics of the pig kidney Na+/glucose cotransporter SGLT2 reveal a common mechanism for SGLT1 and SGLT2. J. Biol. Chem. 271, 32678–32683 (1996).
Jauch, P. & Läuger, P. Electrogenic properties of the sodium–alanine cotransporter in pancreatic acinar cells: II. Comparison with transport models. J. Membr. Biol. 94, 117–127 (1986).
Gunshin, H., Noguchi, T. & Naito, H. Effect of calcium on the zinc uptake by brush border membrane vesicles isolated from the rat small intestine. Agric. Biol. Chem. 55, 2813–2816 (1991).
Casey, J. L. et al. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science 240, 924–928 (1988).
Klausner, R. D., Rouault, T. A. & Harford, J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 72, 19–28 (1993).
Nichols, G. M. & Bacon, B. R. Hereditary hemochromatosis: pathogenesis and clinical features of a common disease. Am. J. Gastroenterol. 84, 851–862 (1989).
Supek, F., Supekova, L., Nelson, H. & Nelson, N. Ayeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl Acad. Sci. USA 93, 5105–5110 (1996).
Hediger, M. A. & Rhoads, D. Molecular physiology of sodium–glucose cotransporters. Physiol. Rev. 74, 993–1026 (1994).
Schaeren-Wiemers, N. & Gerfin-Moser, A. Asingle protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100, 431–440 (1993).
Romero, M. F., Hediger, M. A., Boulpaep, E. L. & Boron, W. F. Expression cloning of the renal electrogenic Na+/HCO3− cotransporter. Nature 387, 409–413 (1997).
Acknowledgements
Nramp cDNA was a generous gift from P. Gros. We thank D. Eide for stimulating discussion. This research was supported by the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunshin, H., Mackenzie, B., Berger, U. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 (1997). https://doi.org/10.1038/41343
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/41343
This article is cited by
-
Mechanisms of Ferritinophagy and Ferroptosis in Diseases
Molecular Neurobiology (2024)
-
The mechanism of ferroptosis and its related diseases
Molecular Biomedicine (2023)
-
Environmental impact on carcinogenesis under BRCA1 haploinsufficiency
Genes and Environment (2023)
-
SLC11A2: a promising biomarker and therapeutic target in ovarian cancer
Scientific Reports (2023)
-
Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney
Nature Reviews Nephrology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.