Abstract
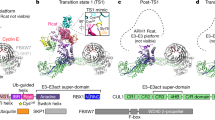
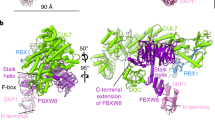
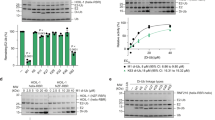
SCF complexes are the largest family of E3 ubiquitin–protein ligases and mediate the ubiquitination of diverse regulatory and signalling proteins. Here we present the crystal structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF complex, which shows that Cul1 is an elongated protein that consists of a long stalk and a globular domain. The globular domain binds the RING finger protein Rbx1 through an intermolecular β-sheet, forming a two-subunit catalytic core that recruits the ubiquitin-conjugating enzyme. The long stalk, which consists of three repeats of a novel five-helix motif, binds the Skp1–F boxSkp2 protein substrate-recognition complex at its tip. Cul1 serves as a rigid scaffold that organizes the Skp1–F boxSkp2 and Rbx1 subunits, holding them over 100 Å apart. The structure suggests that Cul1 may contribute to catalysis through the positioning of the substrate and the ubiquitin-conjugating enzyme, and this model is supported by Cul1 mutations designed to eliminate the rigidity of the scaffold.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998)
Deshaies, R. J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 (1999)
Koepp, D. M., Harper, J. W. & Elledge, S. J. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97, 431–434 (1999)
Strohmaier, H. et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 (2001)
Koepp, D. M. et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 (2001)
Moberg, K. H., Bell, D. W., Wahrer, D. C., Haber, D. A. & Hariharan, I. K. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 413, 311–316 (2001)
Polakis, P. Wnt signaling and cancer. Genes Dev. 14, 1837–1851 (2000)
Slingerland, J. & Pagano, M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J. Cell Physiol. 183, 10–17 (2000)
Latres, E. et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl Acad. Sci. USA 98, 2515–2520 (2001)
Gstaiger, M. et al. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl Acad. Sci. USA 98, 5043–5048 (2001)
Latif, F. et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260, 1317–1320 (1993)
Stebbins, C. E., Kaelin, W. G. Jr & Pavletich, N. P. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 284, 455–461 (1999)
Pickart, C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001)
Joazeiro, C. A. & Weissman, A. M. RING finger proteins: mediators of ubiquitin ligase activity. Cell 102, 549–552 (2000)
Bai, C. et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86, 263–274 (1996)
Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91, 209–219 (1997)
Feldman, R. M., Correll, C. C., Kaplan, K. B. & Deshaies, R. J. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91, 221–230 (1997)
Skowyra, D. et al. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and Rbx1. Science 284, 662–665 (1999)
Kamura, T. et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284, 657–661 (1999)
Ohta, T., Michel, J. J., Schottelius, A. J. & Xiong, Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol. Cell 3, 535–541 (1999)
Seol, J. H. et al. Cdc53/cullin and the essential Hrt1 RING-H2 subunit of SCF define a ubiquitin ligase module that activates the E2 enzyme Cdc34. Genes Dev. 13, 1614–1626 (1999)
Kipreos, E. T. & Pagano, M. The F-box protein family. Genome Biol. 1, 3002.1–3002.7 (2000)
Ganoth, D. et al. The cell-cycle regulatory protein Cks1 is required for SCF (Skp2)-mediated ubiquitinylation of p27. Nature Cell Biol. 3, 321–324 (2001)
Spruck, C. et al. A CDK-independent function of mammalian Cks1. Targeting of SCF (Skp2) to the CDK inhibitor p27 (Kip1). Mol. Cell 7, 639–650 (2001)
Kipreos, E. T., Lander, L. E., Wing, J. P., He, W. W. & Hedgecock, E. M. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell 85, 829–839 (1996)
Ivan, M. & Kaelin, W. G. Jr The von Hippel-Lindau tumor suppressor protein. Curr. Opin. Genet. Dev. 11, 27–34 (2001)
Zachariae, W. et al. Mass spectrometric analysis of the anaphase-promoting complex from yeast: identification of a subunit related to cullins. Science 279, 1216–1219 (1998)
Yu, H. et al. Identification of a cullin homology region in a subunit of the anaphase-promoting complex. Science 279, 1219–1222 (1998)
Schulman, B. A. et al. Insights into SCF ubiquitin ligases from the structure of the Skp1–Skp2 complex. Nature 408, 381–386 (2000)
Rice, L. M. & Brunger, A. T. Crystal structure of the vesicular transport protein Sec17: implications for SNAP function in SNARE complex disassembly. Mol. Cell 4, 85–95 (1999)
Mathias, N. et al. Cdc53p acts in concert with Cdc4p and Cdc34p to control the G1-to-S-phase transition and identifies a conserved family of proteins. Mol. Cell Biol. 16, 6634–6643 (1996)
Grossberger, R. et al. Characterization of the DOC1/APC10 subunit of the yeast and the human anaphase-promoting complex. J. Biol. Chem. 274, 14500–14507 (1999)
Zheng, N., Wang, P., Jeffrey, P. D. & Pavletich, N. P. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell 102, 533–539 (2000)
Chen, A. et al. The conserved RING-H2 finger of ROC1 is required for ubiquitin ligation. J. Biol. Chem. 275, 15432–15439 (2000)
Read, M. A. et al. Nedd8 modification of cul-1 activates SCF (beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol. Cell Biol. 20, 2326–2333 (2000)
Morimoto, M., Nishida, T., Honda, R. & Yasuda, H. Modification of cullin-1 by ubiquitin-like protein Nedd8 enhances the activity of SCF (skp2) toward p27 (kip 1). Biochem. Biophys. Res. Commun. 270, 1093–1096 (2000)
Kawakami, T. et al. NEDD8 recruits E2-ubiquitin to SCF E3 ligase. Embo J. 20, 4003–4012 (2001)
Wu, K., Chen, A., Tan, P. & Pan, Z. Q. The Nedd8-conjugated ROC1-CUL1 core ubiquitin ligase utilizes Nedd8 charged surface residues for efficient polyubiquitin chain assembly catalyzed by Cdc34. J. Biol. Chem. 277, 516–527(2002)
Shirane, M. et al. Down-regulation of p27 (Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J. Biol. Chem. 274, 13886–13893 (1999)
Patton, E. E. et al. Cdc53 is a scaffold protein for multiple Cdc34/Skp1/F-box protein complexes that regulate cell division and methionine biosynthesis in yeast. Genes Dev. 12, 692–705 (1998)
Scherer, D. C., Brockman, J. A., Chen, Z., Maniatis, T. & Ballard, D. W. Signal-induced degradation of l kappa B alpha requires site-specific ubiquitination. Proc. Natl Acad. Sci. USA 92, 11259–11263 (1995)
Stroschein, S. L., Bonni, S., Wrana, J. L. & Luo, K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 15, 2822–2836 (2001)
Otwinowski, Z. & Minor, W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
de la Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for the multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276, 472–494 (1997)
Brunger, A. T. et al. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
CCP4 The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Russo, A. A., Jeffrey, P. D., Patten, A. K., Massague, J. & Pavletich, N. P. Crystal structure of the p27Kip 1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382, 325–331 (1996)
Kraulis, P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991)
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insight from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991)
Acknowledgements
We thank H. Erdument-Bromage for N-terminal sequencing and mass spectroscopic analysis; T. Kamura and Z. Pan for reagents; members of the Pavletich laboratory for discussions; C. Murray for administrative assistance; and the staff of the National Synchrotron Light Source X9B beamline and of the Cornell High Energy Synchrotron Source MacCHESS for help with data collection. B.A.S. was supported by a special fellowship from the Leukemia and Lymphoma Society. This work was supported by the NIH, the Howard Hughes Medical Institute, the Dewitt Wallace Foundation, the Samuel and May Rudin Foundation, the Human Frontier Science Program Organization, the Welch Foundation, the Belfer Foundation and the Irma T. Hirschl Fundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, N., Schulman, B., Song, L. et al. Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 (2002). https://doi.org/10.1038/416703a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416703a
This article is cited by
-
RBX1 regulates PKM alternative splicing to facilitate anaplastic thyroid carcinoma metastasis and aerobic glycolysis by destroying the SMAR1/HDAC6 complex
Cell & Bioscience (2023)
-
S100A6: molecular function and biomarker role
Biomarker Research (2023)
-
14-3-3 proteins regulate cullin 7-mediated Eag1 degradation
Cell & Bioscience (2023)
-
NLRP6 potentiates PI3K/AKT signalling by promoting autophagic degradation of p85α to drive tumorigenesis
Nature Communications (2023)
-
Multiple intermolecular interactions facilitate rapid evolution of essential genes
Nature Ecology & Evolution (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.