Abstract
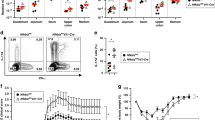
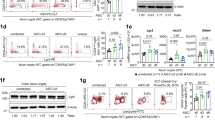
Intestinal epithelial cells (IECs) provide a primary physical barrier against commensal and pathogenic microorganisms in the gastrointestinal (GI) tract, but the influence of IECs on the development and regulation of immunity to infection is unknown1. Here we show that IEC-intrinsic IκB kinase (IKK)-β-dependent gene expression is a critical regulator of responses of dendritic cells and CD4+ T cells in the GI tract. Mice with an IEC-specific deletion of IKK-β show a reduced expression of the epithelial-cell-restricted cytokine thymic stromal lymphopoietin in the intestine and, after infection with the gut-dwelling parasite Trichuris, fail to develop a pathogen-specific CD4+ T helper type 2 (TH2) response and are unable to eradicate infection. Further, these animals show exacerbated production of dendritic-cell-derived interleukin-12/23p40 and tumour necrosis factor-α, increased levels of CD4+ T-cell-derived interferon-γ and interleukin-17, and develop severe intestinal inflammation. Blockade of proinflammatory cytokines during Trichuris infection ablates the requirement for IKK-β in IECs to promote CD4+ TH2 cell-dependent immunity, identifying an essential function for IECs in tissue-specific conditioning of dendritic cells and limiting type 1 cytokine production in the GI tract. These results indicate that the balance of IKK-β-dependent gene expression in the intestinal epithelium is crucial in intestinal immune homeostasis by promoting mucosal immunity and limiting chronic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nagler-Anderson, C. Man the barrier! Strategic defences in the intestinal mucosa. Nature Rev. Immunol. 1, 59–67 (2001)
Neish, A. S. et al. Prokaryotic regulation of epithelial responses by inhibition of IκBα ubiquitination. Science 289, 1560–1563 (2000)
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunol. 6, 507–514 (2005)
Karin, M., Lawrence, T. & Nizet, V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124, 823–835 (2006)
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004)
Bethony, J. et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367, 1521–1532 (2006)
Cliffe, L. J. & Grencis, R. K. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 57, 255–307 (2004)
Owyang, A. M. et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849 (2006)
Schopf, L. R., Hoffmann, K. F., Cheever, A. W., Urban, J. F. & Wynn, T. A. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168, 2383–2392 (2002)
Artis, D. et al. Differential requirement for NF-κB family members in control of helminth infection and intestinal inflammation. J. Immunol. 169, 4481–4487 (2002)
Tilney, L. G., Connelly, P. S., Guild, G. M., Vranich, K. A. & Artis, D. Adaptation of a nematode parasite to living within the mammalian epithelium. J. Exp. Zoolog. A Comp. Exp. Biol. 303, 927–945 (2005)
Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle. Cell 109 (Suppl.). S81–S96 (2002)
Li, Z. W., Omori, S. A., Labuda, T., Karin, M. & Rickert, R. C. IKKβ is required for peripheral B cell survival and proliferation. J. Immunol. 170, 4630–4637 (2003)
Egan, L. J. et al. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc. Natl Acad. Sci. USA 101, 2452–2457 (2004)
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004)
Chen, L. W. et al. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nature Med. 9, 575–581 (2003)
Artis, D. et al. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl Acad. Sci. USA 101, 13596–13600 (2004)
Zhou, Y. et al. Characterization of a calcium-activated chloride channel as a shared target of Th2 cytokine pathways and its potential involvement in asthma. Am. J. Respir. Cell Mol. Biol. 25, 486–491 (2001)
Kelsall, B. L. & Leon, F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol. Rev. 206, 132–148 (2005)
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001)
Iwasaki, A. & Kelsall, B. L. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1394 (2000)
Iwasaki, A. & Kelsall, B. L. Unique functions of CD11b+, CD8α+, and double-negative Peyer’s patch dendritic cells. J. Immunol. 166, 4884–4890 (2001)
Li, M. et al. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl Acad. Sci. USA 103, 11736–11741 (2006)
May, M. J. et al. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289, 1550–1554 (2000)
Ziegler, S. F. & Liu, Y. J. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nature Immunol. 7, 709–714 (2006)
Weaver, C. T., Harrington, L. E., Mangan, P. R., Gavrieli, M. & Murphy, K. M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 (2006)
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005)
Jang, M. H. et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl Acad. Sci. USA 101, 6110–6115 (2004)
Acknowledgements
We thank C. A. Hunter, E. J. Pearce and E. H. Wilson for critical reading of the manuscript, and J. N. Ihle for the Tpte2-/- mice. This work was supported by the NIH (D.A., L.E. and M.K.), the pilot feasibility program of the NIDDK Diseases Centre (D.A.), and NIAID (A.E.T.) and NCRR (L.D.B.-B.) NIH training grants. M.K. is an American Cancer Society Research Professor. C.Z. is a recipient of a fellowship from the Irvington Institute for Immunological Research. D.A. is the recipient of the Crohn’s and Colitis Foundation of America’s William and Shelby Modell Family Foundation Research Award.
Author Contributions C.Z., A.E.T., B.C.T., F.R.G., L.E., M.K. and D.A. designed the research. C.Z., A.E.T., B.C.T., L.D.B.-B., K.J.G., Y.D., E.A.Y. and D.A. performed the research. F.R.G., L.E., A.D.G., M.J.M. and M.K. contributed new reagents. C.Z., A.E.T., B.C.T., F.R.G, L.E., M.J.M, M.K. and D.A. analysed the data. C.Z. and D.A. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
TITLE
The supplementary information contains Supplementary Figures 1-5 and Legends, Supplementary Methods and Supplementary Notes. Supplementary Figure S1 shows the relative mRNA expression level of IL-4 in mLN of infected IkkbF/F and IkkbΔIEC mice and photomicrographs of histological sections of the caecum of infected IkkbF/F and IkkbΔIEC mice stained for goblet cell mucins. Supplementary Figure S2 shows that barrier function is intact in IkkbF/F and IkkbΔIEC mice. Supplementary Figure S3 shows the activation status of dendritic cell subsets in the mLN of naïve IkkbF/F and IkkbΔIEC mice. Supplementary Figure S4 shows the relative mRNA expression levels of CCL6, CCL20 and CCL28 in naïve or infected IkkbF/F and IkkbΔIEC mice. Supplementary Figure S5 shows photomicrographs of histological sections of the caecum stained for goblet cell mucins and levels of secreted RELMΔ in infected IkkbF/F mice and control immunoglobulin or anti-IL-12/23p40/IFN-Δ treated IkkbΔIEC mice. The Supplementary Methods provide details on the experimental procedures. Supplementary Notes contain additional references. (PDF 2431 kb)
Rights and permissions
About this article
Cite this article
Zaph, C., Troy, A., Taylor, B. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007). https://doi.org/10.1038/nature05590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05590
This article is cited by
-
Inflammatory Bowel Disease: Pathophysiology, Treatment, and Disease Modeling
BioChip Journal (2023)
-
The role of diosgenin in crohn’s disease
Clinical Phytoscience (2022)
-
Enterocyte–innate lymphoid cell crosstalk drives early IFN-γ-mediated control of Cryptosporidium
Mucosal Immunology (2022)
-
METTL3 overexpression aggravates LPS-induced cellular inflammation in mouse intestinal epithelial cells and DSS-induced IBD in mice
Cell Death Discovery (2022)
-
Moving towards a systems-based classification of innate immune-mediated diseases
Nature Reviews Rheumatology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.