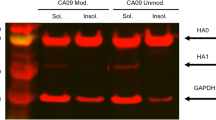
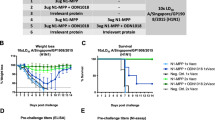
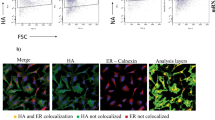
Abstract
Despite substantial improvements, influenza vaccine production—and availability—remain suboptimal. Influenza vaccines based on mRNA may offer a solution as sequence-matched, clinical-grade material could be produced reliably and rapidly in a scalable process, allowing quick response to the emergence of pandemic strains. Here we show that mRNA vaccines induce balanced, long-lived and protective immunity to influenza A virus infections in even very young and very old mice and that the vaccine remains protective upon thermal stress. This vaccine format elicits B and T cell–dependent protection and targets multiple antigens, including the highly conserved viral nucleoprotein, indicating its usefulness as a cross-protective vaccine. In ferrets and pigs, mRNA vaccines induce immunological correlates of protection and protective effects similar to those of a licensed influenza vaccine in pigs. Thus, mRNA vaccines could address substantial medical need in the area of influenza prophylaxis and the broader realm of anti-infective vaccinology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
References
Plotkin, S.A., Orenstein, W.A. & Offit, P.A. Vaccines: Expert Consult (Saunders, 2008).
Fiore, A.E. et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm. Rep. 59, 1–62 (2010).
Doherty, P.C., Turner, S.J., Webby, R.G. & Thomas, P.G. Influenza and the challenge for immunology. Nat. Immunol. 7, 449–455 (2006).
Salomon, R. & Webster, R.G. The influenza virus enigma. Cell 136, 402–410 (2009).
Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A. & Martin, M.A. Fields Virology (Lippincott Williams & Wilkins, 2006).
Tong, S. et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 109, 4269–4274 (2012).
de Jong, J.C., Beyer, W.E., Palache, A.M., Rimmelzwaan, G.F. & Osterhaus, A.D. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J. Med. Virol. 61, 94–99 (2000).
Ulmer, J.B., Valley, U. & Rappuoli, R. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 24, 1377–1383 (2006).
Lambert, L.C. & Fauci, A.S. Influenza vaccines for the future. N. Engl. J. Med. 363, 2036–2044 (2010).
Nabel, G.J. & Fauci, A.S. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat. Med. 16, 1389–1391 (2010).
Forde, G.M. Rapid-response vaccines—does DNA offer a solution? Nat. Biotechnol. 23, 1059–1062 (2005).
Liu, M.A. Immunologic basis of vaccine vectors. Immunity 33, 504–515 (2010).
Thalhamer, J., Weiss, R. & Scheiblhofer, S. Gene Vaccines (Springer, Wien and New York; 2011).
Hoerr, I., Obst, R., Rammensee, H.G. & Jung, G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur. J. Immunol. 30, 1–7 (2000).
Fotin-Mleczek, M. et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 34, 1–15 (2011).
Sebastian, M. et al. Messenger RNA vaccination in NSCLC: findings from a phase I/IIa clinical trial. J. Clin. Oncol. 29 (suppl; abstr 2584) (2011).
Kübler, H. et al. Final analysis of a phase I/IIa study with CV9103, an intradermally administered prostate cancer immunotherapy based on self-adjuvanted mRNA. J. Clin. Oncol. 29 (suppl; abstr 4535) (2011).
Potter, C.W. & Oxford, J.S. Determinants of immunity to influenza infection in man. Br. Med. Bull. 35, 69–75 (1979).
Plotkin, S.A. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47, 401–409 (2008).
Brown, D.M., Dilzer, A.M., Meents, D.L. & Swain, S.L. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 177, 2888–2898 (2006).
Galli, G. et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc. Natl. Acad. Sci. USA 106, 3877–3882 (2009).
Hamada, H. et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182, 3469–3481 (2009).
Wilkinson, T.M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18, 274–280 (2012).
Deng, Y., Yewdell, J.W., Eisenlohr, L.C. & Bennink, J.R. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J. Immunol. 158, 1507–1515 (1997).
Boon, A.C.M. et al. Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model. J. Virol. 84, 7662–7667 (2010).
McMichael, A.J., Gotch, F.M., Noble, G.R. & Beare, P.A. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309, 13–17 (1983).
Ulmer, J.B. et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259, 1745–1749 (1993).
Rimmelzwaan, G.F., Fouchier, R.A.M. & Osterhaus, A.D.M.E. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr. Opin. Biotechnol. 18, 529–536 (2007).
Kistner, O. et al. A whole virus pandemic influenza H1N1 vaccine is highly immunogenic and protective in active immunization and passive protection mouse models. PLoS ONE 5, e9349 (2010).
Chaloupka, I., Schuler, A., Marschall, M. & Meier-Ewert, H. Comparative analysis of six European influenza vaccines. Eur. J. Clin. Microbiol. Infect. Dis. 15, 121–127 (1996).
Belshe, R.B. Translational research on vaccines: influenza as an example. Clin. Pharmacol. Ther. 82, 745–749 (2007).
van der Laan, J.W. et al. Animal models in influenza vaccine testing. Expert Rev. Vaccines 7, 783–793 (2008).
Van Reeth, K., Labarque, G., De Clercq, S. & Pensaert, M. Efficacy of vaccination of pigs with different H1N1 swine influenza viruses using a recent challenge strain and different parameters of protection. Vaccine 19, 4479–4486 (2001).
Pyo, H.-M. et al. Pandemic H1N1 influenza virus-like particles are immunogenic and provide protective immunity to pigs. Vaccine 30, 1297–1304 (2012).
Lefevre, E.A. et al. Immune responses in pigs vaccinated with adjuvanted and non-adjuvanted A(H1N1)pdm/09 influenza vaccines used in human immunization programmes. PLoS ONE 7, e32400 (2012).
Laurent, P.E. et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine 25, 8833–8842 (2007).
Dormitzer, P.R., Ulmer, J.B. & Rappuoli, R. Structure-based antigen design: a strategy for next generation vaccines. Trends Biotechnol. 26, 659–667 (2008).
Johansson, D.X., Ljungberg, K., Kakoulidou, M. & Liljeström, P. Intradermal electroporation of naked replicon RNA elicits strong immune responses. PLoS ONE 7, e29732 (2012).
Fotin-Mleczek, M. et al. Highly potent mRNA based cancer vaccines represent an attractive platform for combination therapies supporting an improved therapeutic effect. J. Gene Med. 14, 428–439 (2012).
Schlake, T. et al. Developing mRNA-vaccine technologies. RNA Biol. (in the press) (2012).
Pascolo, S. Vaccination with messenger RNA. Methods Mol. Med. 127, 23–40 (2006).
Pascolo, S. Vaccination with messenger RNA (mRNA). Handb. Exp. Pharmacol. 221–235 (2008) doi:10.1007/978-3-540-72167-3_11.
Reed, L.J. & Muench, H. A simple method of estimation of fifty percent end points. Am. J. Hyg. 27, 493–497 (1938).
Hai, R. et al. PB1-F2 expression by the 2009 pandemic H1N1 influenza virus has minimal impact on virulence in animal models. J. Virol. 84, 4442–4450 (2010).
Cobbold, S.P., Jayasuriya, A., Nash, A., Prospero, T.D. & Waldmann, H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312, 548–551 (1984).
Lange, E. et al. Pathogenesis and transmission of the novel swine-origin influenza virus A/H1N1 after experimental infection of pigs. J. Gen. Virol. 90, 2119–2123 (2009).
Hoffmann, B. et al. New real-time reverse transcriptase polymerase chain reactions facilitate detection and differentiation of novel A/H1N1 influenza virus in porcine and human samples. Berl. Munch. Tierarztl. Wochenschr. 123, 286–292 (2010).
Acknowledgements
We thank A. Carnitz, A. Möbes, K. Neumann, M. Queiser, N. Schneck, H. Schneider and E. Zirdum for excellent technical assistance and T. Ketterer, T. Mutzke, A. Schmid and their team for production of mRNA vaccines. We thank the following colleagues for graciously providing virus strains: M. Büttner (A/mallard/Bavaria/1/2006 (H5N1)), J. McCauley (A/Vietnam/1194/2004 (H5N1)), S. Becker (A/Regensburg/D6/2009 (H1N1v); received from M. Beer and T. Mettenleiter), O. Haller and G. Kochs (A/HongKong/1/1968 (H3N2)). We thank R. Zinkernagel and B. Schönfisch for critically reading the manuscript. This work was supported by grants from the Federal Ministry for Education and Research (BMBF), Germany (KMU-innovativ, grant no. 0315802 to T.K.) and by the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV), Germany (FSI, project no. 2-43 to L.S.).
Author information
Authors and Affiliations
Contributions
B.P., M.S., A.B.V., E.L., D.V., K.-J.K., L.S. and T.K. conceived the project and designed experiments; B.P., M.S., A.B.V., E.L., B.H., A.T., T.S., D.V. and L.S. performed experiments; B.P., M.S., A.B.V., E.L., B.H., A.T., T.S. and D.V. did data analysis; B.P., M.S., K.-J.K., L.S. and T.K. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
B.P., M.S., D.V., A.T., T.S., K.-J.K. and T.K. are employees of CureVac GmbH, a private company developing RNA-based vaccines and immunotherapeutics. B.P., M.S., K.-J.K., L.S. and T.K. are inventors on two patent applications claiming technical aspects of this work.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5 and Supplementary Figs. 1–5 (PDF 748 kb)
Rights and permissions
About this article
Cite this article
Petsch, B., Schnee, M., Vogel, A. et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol 30, 1210–1216 (2012). https://doi.org/10.1038/nbt.2436
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2436
This article is cited by
-
mRNA vaccines expressing malaria transmission-blocking antigens Pfs25 and Pfs230D1 induce a functional immune response
npj Vaccines (2024)
-
Targeting the hallmarks of aging to improve influenza vaccine responses in older adults
Immunity & Ageing (2023)
-
Seasonal quadrivalent mRNA vaccine prevents and mitigates influenza infection
npj Vaccines (2023)
-
Immunoregulatory nanomedicine for respiratory infections
Nature Reviews Bioengineering (2023)
-
Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus
Signal Transduction and Targeted Therapy (2023)