Abstract
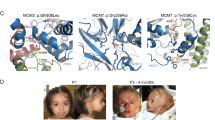
MicroRNAs (miRNAs) are key regulators of gene expression in animals and plants. Studies in a variety of model organisms show that miRNAs modulate developmental processes. To our knowledge, the only hereditary condition known to be caused by a miRNA is a form of adult-onset non-syndromic deafness1, and no miRNA mutation has yet been found to be responsible for any developmental defect in humans. Here we report the identification of germline hemizygous deletions of MIR17HG, encoding the miR-17∼92 polycistronic miRNA cluster, in individuals with microcephaly, short stature and digital abnormalities. We demonstrate that haploinsufficiency of miR-17∼92 is responsible for these developmental abnormalities by showing that mice harboring targeted deletion of the miR-17∼92 cluster phenocopy several key features of the affected humans. These findings identify a regulatory function for miR-17∼92 in growth and skeletal development and represent the first example of an miRNA gene responsible for a syndromic developmental defect in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mencía, A. et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat. Genet. 41, 609–613 (2009).
Fontana, L. et al. Antagomir-17–5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS ONE 3, e2236 (2008).
Hayashita, Y. et al. A polycistronic microRNA cluster, miR-17–92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 65, 9628–9632 (2005).
He, L. et al. A microRNA polycistron as a potential human oncogene. Nature 435, 828–833 (2005).
Mu, P. et al. Genetic dissection of the miR-17∼92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 23, 2806–2811 (2009).
Northcott, P.A. et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog–driven medulloblastomas and induced by N-myc in sonic hedgehog–treated cerebellar neural precursors. Cancer Res. 69, 3249–3255 (2009).
Olive, V. et al. miR-19 is a key oncogenic component of mir-17–92. Genes Dev. 23, 2839–2849 (2009).
Ota, A. et al. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 64, 3087–3095 (2004).
Tagawa, H. & Seto, M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia 19, 2013–2016 (2005).
Uziel, T. et al. The miR-17∼92 cluster collaborates with the sonic hedgehog pathway in medulloblastoma. Proc. Natl. Acad. Sci. USA 106, 2812–2817 (2009).
Ventura, A. et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132, 875–886 (2008).
Celli, J., van Bokhoven, H. & Brunner, H.G. Feingold syndrome: clinical review and genetic mapping. Am. J. Med. Genet. A. 122A, 294–300 (2003).
Feingold, M., Hall, B.D., Lacassie, Y. & Martinez-Frias, M.L. Syndrome of microcephaly, facial and hand abnormalities, tracheoesophageal fistula, duodenal atresia, and developmental delay. Am. J. Med. Genet. 69, 245–249 (1997).
van Bokhoven, H. et al. MYCN haploinsufficiency is associated with reduced brain size and intestinal atresias in Feingold syndrome. Nat. Genet. 37, 465–467 (2005).
Marcelis, C.L. et al. Genotype-phenotype correlations in MYCN-related Feingold syndrome. Hum. Mutat. 29, 1125–1132 (2008).
Firth, H.V. et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 84, 524–533 (2009).
Morales, J.A., Mendizabal, A.P., Vasquez, A.I., Figuera, L.E. & Gonzalez-Garcia, J.R. Interstitial deletion of 13q22→q31: case report and review of the literature. Clin. Dysmorphol. 15, 139–143 (2006).
Quélin, C. et al. Twelve new patients with 13q deletion syndrome: genotype-phenotype analyses in progress. Eur. J. Med. Genet. 52, 41–46 (2009).
Iafrate, A.J. et al. Detection of large-scale variation in the human genome. Nat. Genet. 36, 949–951 (2004).
Kidd, J.M. et al. Mapping and sequencing of structural variation from eight human genomes. Nature 453, 56–64 (2008).
O'Donnell, K.A., Wentzel, E.A., Zeller, K.I., Dang, C.V. & Mendell, J.T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435, 839–843 (2005).
Schulte, J.H. et al. MYCN regulates oncogenic microRNAs in neuroblastoma. Int. J. Cancer 122, 699–704 (2008).
Lovén, J. et al. MYCN-regulated microRNAs repress estrogen receptor-α (ESR1) expression and neuronal differentiation in human neuroblastoma. Proc. Natl. Acad. Sci. USA 107, 1553–1558 (2010).
Chen, X. et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 (2008).
Dews, M. et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat. Genet. 38, 1060–1065 (2006).
Nagy, A. et al. Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr. Biol. 8, 661–664 (1998).
Moens, C.B., Auerbach, A.B., Conlon, R.A., Joyner, A.L. & Rossant, J. A targeted mutation reveals a role for N-myc in branching morphogenesis in the embryonic mouse lung. Genes Dev. 6, 691–704 (1992).
Ota, S., Zhou, Z.Q., Keene, D.R., Knoepfler, P. & Hurlin, P.J. Activities of N-Myc in the developing limb link control of skeletal size with digit separation. Development 134, 1583–1592 (2007).
Stanton, B.R., Perkins, A.S., Tessarollo, L., Sassoon, D.A. & Parada, L.F. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 6, 2235–2247 (1992).
Sawai, S. et al. Defects of embryonic organogenesis resulting from targeted disruption of the N-myc gene in the mouse. Development 117, 1445–1455 (1993).
Mestdagh, P. et al. The miR-17–92 microRNA cluster regulates multiple components of the TGF-β pathway in neuroblastoma. Mol. Cell 40, 762–773 (2010).
Volinia, S. et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 103, 2257–2261 (2006).
Dews, M. et al. The myc-miR-17∼92 axis blunts TGF-β signaling and production of multiple TGF-β-dependent antiangiogenic factors. Cancer Res. 70, 8233–8246 (2010).
Cognet, M. et al. Dissection of the MYCN locus in Feingold syndrome and isolated oesophageal atresia. Eur. J. Hum. Genet. 19, 602–606 (2011).
Masurel-Paulet, A. et al. Delineation of 15q13.3 microdeletions. Clin. Genet. 78, 149–161 (2010).
Acknowledgements
We are thankful to the subjects and their referent doctors for their active participation in this study. We are also particularly thankful to P. Hurlin for generously providing forelimbs of Mycn conditional knockout mouse embryos. This work was supported by grants from the Agence Nationale de la Recherche (ANR grant EvoDevoMut), the Foundation pour la Recherche Médicale (FRM), the Institut National du Cancer-Direction de l'Hospitalisation et de l'Organisation des soins (INCa-DHOS), and the Institut National du Cancer. Work in the laboratory of A.V. was funded by US National Institutes of Health (NIH)-National Cancer Institute (NCI) grant R01CA149707, a Sidney Kimmel Award and a Geoffrey Beene Research Grant. E.Y. is a recipient of the NIH Molecular and Cellular Biology T32 training grant. We thank L. Selleri for her expertise in the phenotypic analysis of miR-17∼92–mutant mice skeletons, L. Legeai-Mallet, A. Pelet and P. Ogrodowski for helpful discussion and technical advice and J. Hollenstein for editing the manuscript.
Author information
Authors and Affiliations
Contributions
L.d.P., P.C., S.C. and M.O. performed subject-related experiments. E.Y. performed the analysis of miR-17∼92 mutant mice, the ChIP experiments and determined miR-17∼92 expression in subjects. J.A.V. determined miR-17∼92 expression in mouse embryos. J.A. and A.V. designed and supervised the project and wrote the manuscript. A.M., M.V., S.L., L.d.P., E.Y. and A.H.-C. provided critical input into project development and manuscript preparation. All other coauthors identified subjects with Feingold syndrome and performed related clinical and laboratory studies (L.F., V.D., A.V.H., D.G., A.G. and S.M.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–3 and Supplementary Note. (PDF 2159 kb)
Rights and permissions
About this article
Cite this article
de Pontual, L., Yao, E., Callier, P. et al. Germline deletion of the miR-17∼92 cluster causes skeletal and growth defects in humans. Nat Genet 43, 1026–1030 (2011). https://doi.org/10.1038/ng.915
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.915
This article is cited by
-
High molecular diagnostic yields and novel phenotypic expansions involving syndromic anorectal malformations
European Journal of Human Genetics (2023)
-
The developmental miR-17–92 cluster and the Sfmbt2 miRNA cluster cannot rescue the abnormal embryonic development generated using obstructive epididymal environment-producing sperm in C57BL/6 J mice
Reproductive Biology and Endocrinology (2022)
-
Dual functions of microRNA-17 in maintaining cartilage homeostasis and protection against osteoarthritis
Nature Communications (2022)
-
miR17~92 restrains pro-apoptotic BIM to ensure survival of haematopoietic stem and progenitor cells
Cell Death & Differentiation (2020)
-
miRNA regulation of social and anxiety-related behaviour
Cellular and Molecular Life Sciences (2020)