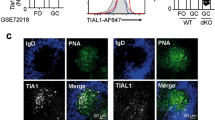
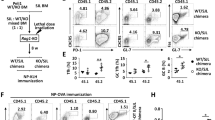
Abstract
Follicular helper T cells (TFH cells) provide critical help to B cells during humoral immune responses. Here we report that mice with T cell–specific deletion of the miR-17∼92 family of microRNAs (miRNAs) had substantially compromised TFH differentiation, germinal-center formation and antibody responses and failed to control chronic viral infection. Conversely, mice with T cell–specific expression of a transgene encoding miR-17∼92 spontaneously accumulated TFH cells and developed a fatal immunopathology. Mechanistically, the miR-17∼92 family controlled the migration of CD4+ T cells into B cell follicles by regulating signaling intensity from the inducible costimulator ICOS and kinase PI(3)K by suppressing expression of the phosphatase PHLPP2. Our findings demonstrate an essential role for the miR-17∼92 family in TFH differentiation and establish PHLPP2 as an important mediator of their function in this process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ambros, V. The functions of animal microRNAs. Nature 431, 350–355 (2004).
Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Bushati, N. & Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 23, 175–205 (2007).
O'Connell, R.M., Rao, D.S., Chaudhuri, A.A. & Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 (2010).
Xiao, C. & Rajewsky, K. MicroRNA control in the immune system: basic principles. Cell 136, 26–36 (2009).
Mendell, J.T. miRiad roles for the miR-17–92 cluster in development and disease. Cell 133, 217–222 (2008).
Ventura, A. et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132, 875–886 (2008).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Johnston, R.J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Nurieva, R.I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Lee, P.P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001).
Fahey, L.M. et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 208, 987–999 (2011).
Harker, J.A., Lewis, G.M., Mack, L. & Zuniga, E.I. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829 (2011).
Ahmed, R. & Oldstone, M.B. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 167, 1719–1724 (1988).
Elsaesser, H., Sauer, K. & Brooks, D.G. IL-21 is required to control chronic viral infection. Science 324, 1569–1572 (2009).
Fröhlich, A. et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324, 1576–1580 (2009).
Linterman, M.A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Nurieva, R.I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008).
Yi, J.S., Du, M. & Zajac, A.J. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324, 1572–1576 (2009).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Barber, D.L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Xiao, C. et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nat. Immunol. 9, 405–414 (2008).
Linterman, M.A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Simpson, N. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62, 234–244 (2010).
Vinuesa, C.G. et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435, 452–458 (2005).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Baumjohann, D., Okada, T. & Ansel, K.M. Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 187, 2089–2092 (2011).
Xu, H. et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 496, 523–527 (2013).
Gigoux, M. et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA 106, 20371–20376 (2009).
Rolf, J. et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J. Immunol. 185, 4042–4052 (2010).
Jiang, P., Rao, E.Y., Meng, N., Zhao, Y. & Wang, J.J. MicroRNA-17–92 significantly enhances radioresistance in human mantle cell lymphoma cells. Radiat. Oncol. 5, 100 (2010).
Choi, Y.S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Jiang, S. et al. Molecular dissection of the miR-17–92 cluster's critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood 118, 5487–5497 (2011).
Lesche, R. et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32, 148–149 (2002).
Ahmed, R., Salmi, A., Butler, L.D., Chiller, J.M. & Oldstone, M.B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984).
Suto, A. et al. Development and characterization of IL-21-producing CD4+ T cells. J. Exp. Med. 205, 1369–1379 (2008).
Lu, K.T. et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity 35, 622–632 (2011).
Acknowledgements
We thank Y. Zheng, C. Fine and B. Rezner for technical assistance; I. Tobias and A. Newton (University of California, San Diego) for the plasmid pcDNA3-HA-PHLPP2; C.H. Kim, E. Stone, S.M. Hedrick, K. Sauer and members of Xiao laboratory for discussion and critical reading of the manuscript. Supported by the Pew Charitable Trusts, the Cancer Research Institute, the Lupus Research Institute, the American Heart Association (11POST7430106 to J.R.T.), the US National Institutes of Health (R01AI019484 to M.B.A.O. and R01AI087634 to C.X.) and National Natural Science Foundation of China (81161120405 to H.Q.).
Author information
Authors and Affiliations
Contributions
C.X., S.G.K., W.-H.L. and J.R.T. conceived of and designed the project; P.L. did immunostaining and some PHLPP2-related experiments under the supervision of H.Q.; H.W.L. did in vitro TFH-differentiation experiments under the supervision of E.V.; S.G.K., W.-H.L., J.R.T., H.Y.J., J.S. and D.F. did all other experiments under the supervision of C.X., J.R.T. and M.B.A.O.; and S.G.K., W.-H.L., J.R.T. and C.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 12001 kb)
Rights and permissions
About this article
Cite this article
Kang, S., Liu, WH., Lu, P. et al. MicroRNAs of the miR-17∼92 family are critical regulators of TFH differentiation. Nat Immunol 14, 849–857 (2013). https://doi.org/10.1038/ni.2648
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2648
This article is cited by
-
Dhx33 promotes B-cell growth and proliferation by controlling activation-induced rRNA upregulation
Cellular & Molecular Immunology (2023)
-
ADRB2 expression predicts the clinical outcomes and is associated with immune cells infiltration in lung adenocarcinoma
Scientific Reports (2022)
-
miR-20a suppresses Treg differentiation by targeting Map3k9 in experimental autoimmune encephalomyelitis
Journal of Translational Medicine (2021)
-
The ubiquitin ligase Peli1 inhibits ICOS and thereby Tfh-mediated immunity
Cellular & Molecular Immunology (2021)
-
Three paralogous clusters of the miR-17~92 family of microRNAs restrain IL-12-mediated immune defense
Cellular & Molecular Immunology (2021)