Abstract
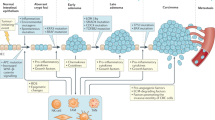
Inflammation is emerging as one of the hallmarks of cancer, yet its role in most tumors remains unclear. Whereas a minority of solid tumors are associated with overt inflammation, long-term treatment with non-steroidal anti-inflammatory drugs is remarkably effective in reducing cancer rate and death. This indicates that inflammation might have many as-yet-unrecognized facets, among which an indolent course might be far more prevalent than previously appreciated. In this Review, we explore the various inflammatory processes underlying the development and progression of colorectal cancer and discuss anti-inflammatory means for its prevention and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan, D. & Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Aggarwal, B.B., Vijayalekshmi, R.V. & Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res. 15, 425–430 (2009).
Balkwill, F.R. & Mantovani, A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin. Cancer Biol. 22, 33–40 (2012).
Rowan, A.J. et al. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”. Proc. Natl. Acad. Sci. USA 97, 3352–3357 (2000).
Groden, J. et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66, 589–600 (1991).
Jess, T., Frisch, M. & Simonsen, J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin. Gastroenterol. Hepatol. 11, 43–48 (2013).
Labayle, D. et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 101, 635–639 (1991).
Burn, J. et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 378, 2081–2087 (2011).
Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Zhang, K., Hornef, M.W. & Dupont, A. The intestinal epithelium as guardian of gut barrier integrity. Cell. Microbiol. 17, 1561–1569 (2015).
Tlaskalová-Hogenová, H. et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 8, 110–120 (2011).
Tsiaoussis, G.I., Assimakopoulos, S.F., Tsamandas, A.C., Triantos, C.K. & Thomopoulos, K.C. Intestinal barrier dysfunction in cirrhosis: Current concepts in pathophysiology and clinical implications. World J Hepatol 7, 2058–2068 (2015).
Kiesslich, R. et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 61, 1146–1153 (2012).
Salim, S.Y. & Soderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 362–381 (2011).
Velcich, A. et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295, 1726–1729 (2002).
Dunn, G.P., Koebel, C.M. & Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Saleh, M. & Trinchieri, G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat. Rev. Immunol. 11, 9–20 (2011).
Lin, W.W. & Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 117, 1175–1183 (2007).
Siegal, F.P. et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 (1999).
Schoggins, J.W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Kemp, M.G. & Sancar, A. DNA excision repair: where do all the dimers go? Cell Cycle 11, 2997–3002 (2012).
Sharma, S., Fitzgerald, K.A., Cancro, M.P. & Marshak-Rothstein, A. Nucleic acid-sensing receptors: rheostats of autoimmunity and autoinflammation. J. Immunol. 195, 3507–3512 (2015).
Härtlova, A. et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity 42, 332–343 (2015).
Zhu, Q. et al. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J. Immunol. 193, 4779–4782 (2014).
Dihlmann, S. et al. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int. J. Cancer 135, 2387–2396 (2014).
Hou, J. et al. Hepatic RIG-I predicts survival and interferon-α therapeutic response in hepatocellular carcinoma. Cancer Cell 25, 49–63 (2014).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Hoque, R. et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 141, 358–369 (2011).
Pétrilli, V., Dostert, C., Muruve, D.A. & Tschopp, J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19, 615–622 (2007).
Vanaja, S.K., Rathinam, V.A. & Fitzgerald, K.A. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 25, 308–315 (2015).
Kanarek, N. et al. Critical role for IL-1β in DNA damage-induced mucositis. Proc. Natl. Acad. Sci. USA 111, E702–E711 (2014).
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443 (2015).
Harrison, O.J. et al. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3+ Treg cell function in the intestine. Mucosal Immunol. 8, 1226–1236 (2015).
Sellin, M.E., Maslowski, K.M., Maloy, K.J. & Hardt, W.D. Inflammasomes of the intestinal epithelium. Trends Immunol. 36, 442–450 (2015).
Hu, B. et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl. Acad. Sci. USA 107, 21635–21640 (2010).
Man, S.M. et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell 162, 45–58 (2015).
Rommereim, L.M. & Subramanian, N. AIMing 2 Curtail Cancer. Cell 162, 18–20 (2015).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl. Acad. Sci. USA 108, 9601–9606 (2011).
Khor, B., Gardet, A. & Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Muise, A.M. et al. Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn's disease. Gut 58, 1121–1127 (2009).
Sabath, E. et al. Galpha12 regulates protein interactions within the MDCK cell tight junction and inhibits tight-junction assembly. J. Cell Sci. 121, 814–824 (2008).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Keshavarzian, A. et al. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology 103, 177–185 (1992).
Rioux, J.D. et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 39, 596–604 (2007).
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39, 207–211 (2007).
McCarroll, S.A. et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat. Genet. 40, 1107–1112 (2008).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Cattin, A.L. et al. Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol. Cell. Biol. 29, 6294–6308 (2009).
Villani, A.C. et al. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat. Genet. 41, 71–76 (2009).
Allen, I.C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Zaki, M.H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Adolph, T.E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Biswas, A. et al. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc. Natl. Acad. Sci. USA 107, 14739–14744 (2010).
Porter, E.M., Bevins, C.L., Ghosh, D. & Ganz, T. The multifaceted Paneth cell. Cellular and molecular life sciences. Cell. Mol. Life Sci. 59, 156–170 (2002).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 12, 715–723 (2011).
Karin, M. & Ben-Neriah, Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 (2000).
Shaked, H. et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc. Natl. Acad. Sci. USA 109, 14007–14012 (2012).
Greten, F.R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
West, N.R., McCuaig, S., Franchini, F. & Powrie, F. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 15, 615–629 (2015).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Bollrath, J. et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15, 91–102 (2009).
Rokavec, M. et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 124, 1853–1867 (2014).
Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2, 364–371 (2002).
Reich, K. et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 366, 1367–1374 (2005).
Moller, D.E. Potential role of TNF-α in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 11, 212–217 (2000).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Pribluda, A. et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell 24, 242–256 (2013).
Tilstra, J.S. et al. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Invest. 122, 2601–2612 (2012).
Schwitalla, S. et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell 23, 93–106 (2013).
Cooks, T. et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 23, 634–646 (2013).
Rodrigues, N.R. et al. p53 mutations in colorectal cancer. Proc. Natl. Acad. Sci. USA 87, 7555–7559 (1990).
Brentnall, T.A. et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology 107, 369–378 (1994).
Tjalsma, H., Boleij, A., Marchesi, J.R. & Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat. Rev. Microbiol. 10, 575–582 (2012).
Parsonnet, J. et al. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325, 1127–1131 (1991).
The EUROGAST Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet 341, 1359–1362 (1993).
Wotherspoon, A.C., Ortiz-Hidalgo, C., Falzon, M.R. & Isaacson, P.G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338, 1175–1176 (1991).
Wotherspoon, A.C. et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342, 575–577 (1993).
Zhang, Y.J. et al. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 16, 7493–7519 (2015).
O'Hara, A.M. & Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 7, 688–693 (2006).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 (2013).
Sears, C.L. & Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host Microbe 15, 317–328 (2014).
Grivennikov, S.I. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258 (2012).
Wang, K. et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity 41, 1052–1063 (2014).
Lévy, J. et al. Intestinal inhibition of Atg7 prevents tumour initiation through a microbiome-influenced immune response and suppresses tumour growth. Nat. Cell Biol 17, 1062–1073 (2015).
Kirchberger, S. et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210, 917–931 (2013).
Mathew, R., Karantza-Wadsworth, V. & White, E. Role of autophagy in cancer. Nat. Rev. Cancer 7, 961–967 (2007).
Vitale, I., Manic, G., Dandrea, V. & De Maria, R. Role of autophagy in the maintenance and function of cancer stem cells. Int. J. Dev. Biol. 59, 95–108 (2015).
Jiang, X., Overholtzer, M. & Thompson, C.B. Autophagy in cellular metabolism and cancer. J. Clin. Invest. 125, 47–54 (2015).
Ijssennagger, N. et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. USA 112, 10038–10043 (2015).
Abdulamir, A.S., Hafidh, R.R. & Bakar, F.A. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol. Cancer 9, 249 (2010).
Wu, S., Lim, K.C., Huang, J., Saidi, R.F. & Sears, C.L. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 95, 14979–14984 (1998).
Housseau, F. & Sears, C.L. Enterotoxigenic Bacteroides fragilis (ETBF)-mediated colitis in Min (Apc+/−) mice: a human commensal-based murine model of colon carcinogenesis. Cell Cycle 9, 3–5 (2010).
Wu, S. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022 (2009).
Bondar, T. & Medzhitov, R. The origins of tumor-promoting inflammation. Cancer Cell 24, 143–144 (2013).
Amit, S. et al. Axin-mediated CKI phosphorylation of β-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16, 1066–1076 (2002).
Elyada, E. et al. CKIα ablation highlights a critical role for p53 in invasiveness control. Nature 470, 409–413 (2011).
Bos, C.L. et al. Effect of aspirin on the Wnt/β-catenin pathway is mediated via protein phosphatase 2A. Oncogene 25, 6447–6456 (2006).
Waddell, W.R., Ganser, G.F., Cerise, E.J. & Loughry, R.W. Sulindac for polyposis of the colon. Am. J. Surg. 157, 175–179 (1989).
Beazer-Barclay, Y. et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis 17, 1757–1760 (1996).
Thun, M.J., Namboodiri, M.M. & Heath, C.W. Jr. Aspirin use and reduced risk of fatal colon cancer. N. Engl. J. Med. 325, 1593–1596 (1991).
Sandler, R.S. et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med. 348, 883–890 (2003).
Ng, K. et al. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J. Natl. Cancer Inst. 107, 345 (2015).
Rothwell, P.M. et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31–41 (2011).
Fraser, D.M., Sullivan, F.M., Thompson, A.M. & McCowan, C. Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study. Br. J. Cancer 111, 623–627 (2014).
Streicher, S.A., Yu, H., Lu, L., Kidd, M.S. & Risch, H.A. Case-control study of aspirin use and risk of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 23, 1254–1263 (2014).
Hawkey, C.J. COX-2 inhibitors. Lancet 353, 307–314 (1999).
Williams, C.S., Shattuck-Brandt, R.L. & DuBois, R.N. The role of COX-2 in intestinal cancer. Expert Opin. Investig. Drugs 8, 1–12 (1999).
Holmes, M.D. et al. COX-2 expression predicts worse breast cancer prognosis and does not modify the association with aspirin. Breast Cancer Res. Treat. 130, 657–662 (2011).
Cascinu, S. et al. COX-2 and NF-κB overexpression is common in pancreatic cancer but does not predict for COX-2 inhibitors activity in combination with gemcitabine and oxaliplatin. Am. J. Clin. Oncol. 30, 526–530 (2007).
Han, S.L., Tang, H.J., Hua, Y.W., Ji, S.Q. & Lin, D.X. Expression of COX-2 in stomach cancers and its relation to their biological features. Dig. Surg. 20, 107–114 (2003).
Kömhoff, M. et al. Enhanced expression of cyclooxygenase-2 in high grade human transitional cell bladder carcinomas. Am. J. Pathol. 157, 29–35 (2000).
Achiwa, H. et al. Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. Clin. Cancer Res. 5, 1001–1005 (1999).
Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231, 232–235 (1971).
Sheng, H., Shao, J., Washington, M.K. & DuBois, R.N. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 276, 18075–18081 (2001).
Williams, C.S., Mann, M. & DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18, 7908–7916 (1999).
Zelenay, S. et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015).
Herfs, M., Hubert, P. & Delvenne, P. Epithelial metaplasia: adult stem cell reprogramming and (pre)neoplastic transformation mediated by inflammation? Trends Mol. Med. 15, 245–253 (2009).
Liu, X.H. et al. Prostaglandin E2 modulates components of the Wnt signaling system in bone and prostate cancer cells. Biochem. Biophys. Res. Commun. 394, 715–720 (2010).
Liao, X. et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N. Engl. J. Med. 367, 1596–1606 (2012).
Domingo, E. et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J. Clin. Oncol. 31, 4297–4305 (2013).
Deisseroth, A. et al. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin. Cancer Res. 21, 950–954 (2015).
Karsdal, M.A. et al. IL-6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti-tumor necrosis factor therapy: biochemical marker analysis of bone metabolism in the tocilizumab RADIATE study (NCT00106522). Semin. Arthritis Rheum. 42, 131–139 (2012).
Heinrich, P.C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Putoczki, T.L. et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24, 257–271 (2013).
Peyrin-Biroulet, L. Anti-TNF therapy in inflammatory bowel diseases: a huge review. Minerva Gastroenterol. Dietol. 56, 233–243 (2010).
Huang, E.S., Strate, L.L., Ho, W.W., Lee, S.S. & Chan, A.T. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am. J. Med. 124, 426–433 (2011).
Biffi, A. et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 75, 693–698 (2010).
Bruns, H. et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J. Clin. Invest. 119, 1167–1177 (2009).
Ali, T. et al. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc. Patient Saf. 5, 79–99 (2013).
Ordonez, M.E., Farraye, F.A. & Di Palma, J.A. Endemic fungal infections in inflammatory bowel disease associated with anti-TNF antibody therapy. Inflamm. Bowel Dis. 19, 2490–2500 (2013).
Mercer, L.K. et al. The influence of anti-TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: longitudinal results from the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 71, 869–874 (2012).
Hoshi, D. et al. Incidence of serious respiratory infections in patients with rheumatoid arthritis treated with tocilizumab. Mod. Rheum. 22, 122–127 (2012).
Galon, J. et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J. Pathol. 232, 199–209 (2014).
Tosolini, M. et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 71, 1263–1271 (2011).
Le, D.T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Cole, B.F. et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J. Natl. Cancer Inst. 101, 256–266 (2009).
Burn, J. et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev. Res. (Phila.) 4, 655–665 (2011).
https://clinicaltrials.gov/ct2/show/NCT00565708?term=NCT00565708&rank=1.
https://clinicaltrials.gov/ct2/show/NCT02301286?term=NCT02301286&rank=1.
Ait Ouakrim, D. et al. Aspirin, ibuprofen, and the risk of colorectal cancer in Lynch syndrome. J. Natl. Cancer Inst. 107, djv170 (2015).
Steinbach, G. et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 342, 1946–1952 (2000).
Bertagnolli, M.M. et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev. Res. (Phila.) 2, 310–321 (2009).
https://clinicaltrials.gov/ct2/show/NCT01150045?term=NCT01150045&rank=1.
https://clinicaltrials.gov/ct2/show/NCT00159484?term=NCT00159484&rank=1.
https://clinicaltrials.gov/ct2/show/NCT00033371?term=NCT00033371&rank=1.
https://clinicaltrials.gov/ct2/show/NCT01483144?term=NCT01483144&rank=1.
https://clinicaltrials.gov/ct2/show/NCT02060188?term=NCT02060188&rank=1.
Angevin, E. et al. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer Res. 20, 2192–2204 (2014).
https://clinicaltrials.gov/ct2/show/NCT01458574?term=NCT01458574&rank=1.
https://clinicaltrials.gov/ct2/show/NCT01470599?term=NCT01470599&rank=1.
Dinarello, C.A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 29, 317–329 (2010).
Wang, Y. et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 7, 1106–1115 (2014).
Xiao, H. et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity 26, 461–475 (2007).
https://clinicaltrials.gov/ct2/show/NCT02090101?term=NCT02090101&rank=1.
Pitzalis, C., Jones, G.W., Bombardieri, M. & Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14, 447–462 (2014).
Dieu-Nosjean, M.C., Goc, J., Giraldo, N.A., Sautes-Fridman, C. & Fridman, W.H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580 (2014).
Coppola, D. et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am. J. Pathol. 179, 37–45 (2011).
Finkin, S. et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 16, 1235–1244 (2015).
Kaplan, K.B. et al. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat. Cell Biol. 3, 429–432 (2001).
Peltomäki, P. Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum. Mol. Genet. 10, 735–740 (2001).
Ferguson, B.J., Mansur, D.S., Peters, N.E., Ren, H. & Smith, G.L. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. eLife 1, e00047 (2012).
Kondo, T. et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc. Natl. Acad. Sci. USA 110, 2969–2974 (2013).
Goodwin, A.C. et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 108, 15354–15359 (2011).
Bergounioux, J. et al. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium's epithelial niche. Cell Host Microbe 11, 240–252 (2012).
Cuevas-Ramos, G. et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. USA 107, 11537–11542 (2010).
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
Acknowledgements
Supported by Israeli Science Foundation Centers of Excellence, the European Research Council Framework Programme 7 (294390 PICHO), The Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the I-CORE program of the Israel Science Foundation (41/11) and the Israel Cancer Research Fund (Y.B.-N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lasry, A., Zinger, A. & Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat Immunol 17, 230–240 (2016). https://doi.org/10.1038/ni.3384
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3384
This article is cited by
-
WAY-262611 ameliorates the inflammatory bowel disease by activating Wnt/β-catenin pathway
In Vitro Cellular & Developmental Biology - Animal (2024)
-
27-Hydroxycholesterol activates the GSK-3β/β-catenin signaling pathway resulting in intestinal fibrosis by inducing oxidative stress: effect of dietary interventions
Inflammation Research (2024)
-
TNFα-induced IDH1 hyperacetylation reprograms redox homeostasis and promotes the chemotherapeutic sensitivity
Oncogene (2023)
-
Gut microbiota in colorectal cancer development and therapy
Nature Reviews Clinical Oncology (2023)
-
Emerging glyco-risk prediction model to forecast response to immune checkpoint inhibitors in colorectal cancer
Journal of Cancer Research and Clinical Oncology (2023)