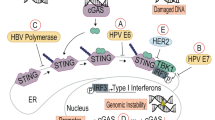
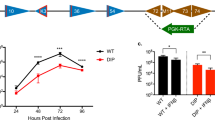
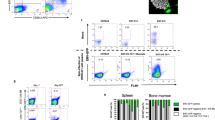
Abstract
Retroviruses evolve rapidly to avoid the immune response of the infected host. We show here that the wild-type mouse mammary tumor virus MMTV(C3H) persisted indefinitely in C3H/HeN mice. However, it was rapidly lost in mice of the closely related C3H/HeJ strain and was replaced by a virus recombinant with an endogenous Mtv provirus. Maintenance of the wild-type virus was dependent on Toll-like receptor-4 (TLR4) signaling, which triggered production of the immunosuppressive cytokine interleukin-10. In the presence of mutant TLR4 in C3H/HeJ mice, wild-type virus was eliminated by the cytotoxic immune response, promoting selection of the immune escape recombinant MMTV variants. Thus, subversion of the innate immune system is yet another survival strategy used by retroviruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tortorella, D., Gewurz, B.E., Furman, M.H., Schust, D.J. & Ploegh, H.L. Viral subversion of the immune system. Annu. Rev. Immunol. 18, 861–926 (2000).
Bowie, A. et al. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97, 10162–10167 (2000).
Preston, B.D. & Dougherty, J.P. Mechanisms of retroviral mutation. Trends Microbiol. 4, 16–21 (1996).
Hu, W.S. & Temin, H.M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc. Natl. Acad. Sci. USA 87, 1556–1560 (1990).
Domingo, E. & Holland, J.J. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51, 151–178 (1997).
Outzen, H.C., Corrow, D. & Shultz, L.D. Attenuation of exogenous murine mammary tumor virus virulence in the C3H/HeJ mouse substrain bearing the Lps mutation. J. Natl. Cancer Inst. 75, 917–923 (1985).
Hook, L.M., Agafonova, Y., Ross, S.R., Turner, S.J. & Golovkina, T.V. Genetics of mouse mammary tumor virus-induced mammary tumors: linkage of tumor induction to the gag gene. J. Virol. 74, 8876–8883 (2000).
Coffin, J.M. Retroviridae: the viruses and their replication. Fundamental Virology (eds. Fields, B.N., Howley, P.M. & Knipe, D.M.) 763–844 (Raven Press, New York, NY, 1996).
MacDonald, H.R. et al. T-cell reactivity and tolerance to Mlsa-encoded antigens. Immunol. Rev. 107, 89–108 (1989).
Held, H. et al. Superantigen-reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J. Exp. Med. 177, 359–366 (1993).
Acha-Orbea, H. & MacDonald, H.R. Superantigens of mouse mammary tumor virus. Annu. Rev. Immunol. 13, 459–486 (1995).
Golovkina, T.V., Dudley, J.P. & Ross, S.R. B and T cells are required for mouse mammary tumor virus spread within the mammary gland. J. Immunol. 161, 2375–2382 (1998).
Golovkina, T.V., Jaffe, A. & Ross, S.R. Coexpression of exogenous and endogenous mouse mammary tumor virus RNA in vivo results in viral recombination and broadens the virus host range. J. Virol. 68, 5019–5026 (1994).
Golovkina, T.V., Dudley, J.P., Jaffe, A.B. & Ross, S.R. Mouse mammary tumor viruses with functional superantigen genes are selected during in vivo infection. Proc. Natl. Acad. Sci. USA 92, 4828–4832 (1995).
Marrack, P., Kushnir, E. & Kappler, J. A maternally inherited superantigen encoded by mammary tumor virus. Nature 349, 524–526 (1991).
Golovkina, T.V., Chervonsky, A.V., Dudley, J.P. & Ross, S.R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell 69, 637–645 (1992).
Held, W. et al. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell 74, 529–540 (1993).
Glode, L.M. & Rosenstreich, D.L. Genetic control of B cell activation by bacterial lipopolysaccharide is mediated by multiple distinct genes or alleles. J. Immunol. 117, 2061–2066 (1976).
Vogel, S.N. et al. Cutting edge: functional characterization of the effect of the C3H/HeJ defect in mice that lack an Lpsn gene: in vivo evidence for a dominant negative mutation. J. Immunol. 162, 5666–5670 (1999).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Hoshino, K. et al. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162, 3749–3752 (1999).
Qureshi, S.T. et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189, 615–625 (1999).
Pucillo, C., Cepeda, R. & Hodes, R.J. Expression of a MHC class II transgene determines both superantigenicity and susceptibility to mammary tumor virus infection. J. Exp. Med. 178, 1441–1445 (1993).
Koller, B.H., Marrack, P., Kappler, J.W. & Smithies, O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248, 1227–1230 (1990).
Rassa, J.C., Meyers, J.L., Zhang, Y., Kudaravalli, R. & Ross, S.R. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99, 2281–2286 (2002).
Moore, K.W., de Waal Malefyt, R., Coffman, R.L. & O'Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Sing, A., Roggenkamp, A., Geiger, A.M. & Heesemann, J. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168, 1315–1321 (2002).
Eaton, K.A., Mefford, M. & Thevenot, T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166, 7456–7461 (2001).
Akridge, R.E., Oyafuso, L.K. & Reed, S.G. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J. Immunol. 153, 5782–5789 (1994).
Fleming, S.B., McCaughan, C.A., Andrews, A.E., Nash, A.D. & Mercer, A.A. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J. Virol. 71, 4857–4861 (1997).
Spencer, J.V. et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76, 1285–1292 (2002).
Sing, A. et al. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196, 1017–1024 (2002).
Beutner, U., Kraus, E., Kitamura, D., Rajewsky, K. & Huber, B.T. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J. Exp. Med. 179, 1457–1466 (1994).
Mombaerts, P. et al. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature 360, 225–231 (1992).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A B-cell deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350, 423 (1991).
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992).
Koller, B.H., Marrack, P., Kappler, J.W. & Smithies, O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science 248, 1227–1230 (1990).
Mach, N. et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 60, 3239–3246 (2000).
Acknowledgements
Supported by US National Institutes of Health grants (to T.V.G. and The Jackson Laboratory), an award from the Hillsdale Foundation (to T.V.G.) and a grant from The Jackson Laboratory (to T.V.G.). We thank D. Roopenian and S. Ross for helpful discussions and S. Williamson for the artwork. This article is dedicated to the memory of Charles Janeway, Jr.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Jude, B., Pobezinskaya, Y., Bishop, J. et al. Subversion of the innate immune system by a retrovirus. Nat Immunol 4, 573–578 (2003). https://doi.org/10.1038/ni926
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni926
This article is cited by
-
Selective evolution of Toll-like receptors 3, 7, 8, and 9 in bats
Immunogenetics (2017)
-
The influence of commensal bacteria on infection with enteric viruses
Nature Reviews Microbiology (2016)
-
The price of immunity
Nature Immunology (2012)
-
Retroviral Vectors Induce Epigenetic Chromatin Modifications and IL-10 Production in Transduced B Cells via Activation of Toll-like Receptor 2
Molecular Therapy (2011)
-
Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis
Fibrogenesis & Tissue Repair (2010)