Abstract
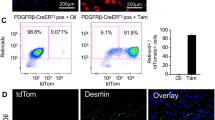
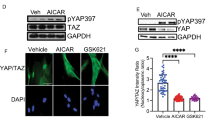
Myofibroblasts are the major source of extracellular matrix components that accumulate during tissue fibrosis, and hepatic stellate cells (HSCs) are believed to be the major source of myofibroblasts in the liver. To date, robust systems to genetically manipulate these cells have not been developed. We report that Cre under control of the promoter of Pdgfrb (Pdgfrb-Cre) inactivates loxP-flanked genes in mouse HSCs with high efficiency. We used this system to delete the gene encoding αv integrin subunit because various αv-containing integrins have been suggested as central mediators of fibrosis in multiple organs. Such depletion protected mice from carbon tetrachloride–induced hepatic fibrosis, whereas global loss of β3, β5 or β6 integrins or conditional loss of β8 integrins in HSCs did not. We also found that Pdgfrb-Cre effectively targeted myofibroblasts in multiple organs, and depletion of the αv integrin subunit using this system was protective in other models of organ fibrosis, including pulmonary and renal fibrosis. Pharmacological blockade of αv-containing integrins by a small molecule (CWHM 12) attenuated both liver and lung fibrosis, including in a therapeutic manner. These data identify a core pathway that regulates fibrosis and suggest that pharmacological targeting of all αv integrins may have clinical utility in the treatment of patients with a broad range of fibrotic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gleizes, P.E. et al. TGF-β latency: biological significance and mechanisms of activation. Stem Cells 15, 190–197 (1997).
Munger, J.S. et al. Latent transforming growth factor-β: structural features and mechanisms of activation. Kidney Int. 51, 1376–1382 (1997).
Munger, J.S. et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Mu, D. et al. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 157, 493–507 (2002).
Annes, J.P., Rifkin, D.B. & Munger, J.S. The integrin αvβ6 binds and activates latent TGFβ3. FEBS Lett. 511, 65–68 (2002).
Aluwihare, P. et al. Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J. Cell Sci. 122, 227–232 (2009).
Wang, B. et al. Role of αvβ6 integrin in acute biliary fibrosis. Hepatology 46, 1404–1412 (2007).
Hahm, K. et al. αv βa6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am. J. Pathol. 170, 110–125 (2007).
Ma, L.J. et al. Transforming growth factor-β-dependent and -independent pathways of induction of tubulointerstitial fibrosis in β6−/− mice. Am. J. Pathol. 163, 1261–1273 (2003).
Breuss, J.M., Gillett, N., Lu, L., Sheppard, D. & Pytela, R. Restricted distribution of integrin β6 mRNA in primate epithelial tissues. J. Histochem. Cytochem. 41, 1521–1527 (1993).
Breuss, J.M. et al. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 108, 2241–2251 (1995).
Shi, M. et al. Latent TGF-β structure and activation. Nature 474, 343–349 (2011).
Wipff, P.J., Rifkin, D.B., Meister, J.J. & Hinz, B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323 (2007).
Munger, J.S., Harpel, J.G., Giancotti, F.G. & Rifkin, D.B. Interactions between growth factors and integrins: latent forms of transforming growth factor-β are ligands for the integrin αvβ1 . Mol. Biol. Cell 9, 2627–2638 (1998).
Asano, Y. et al. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J. Immunol. 175, 7708–7718 (2005).
Asano, Y., Ihn, H., Yamane, K., Jinnin, M. & Tamaki, K. Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am. J. Pathol. 168, 499–510 (2006).
Friedman, S.L. & Arthur, M.J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J. Clin. Invest. 84, 1780–1785 (1989).
Pinzani, M., Gesualdo, L., Sabbah, G.M. & Abboud, H.E. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J. Clin. Invest. 84, 1786–1793 (1989).
Wong, L., Yamasaki, G., Johnson, R.J. & Friedman, S.L. Induction of β-platelet–derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J. Clin. Invest. 94, 1563–1569 (1994).
Pinzani, M. et al. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am. J. Pathol. 148, 785–800 (1996).
Ikura, Y. et al. Expression of platelet-derived growth factor and its receptor in livers of patients with chronic liver disease. J. Gastroenterol. 32, 496–501 (1997).
Coin, P.G. et al. Lipopolysaccharide up-regulates platelet-derived growth factor (PDGF) α-receptor expression in rat lung myofibroblasts and enhances response to all PDGF isoforms. J. Immunol. 156, 4797–4806 (1996).
Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 15, 255–273 (2004).
Chen, Y.T. et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80, 1170–1181 (2011).
Foo, S.S. et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161–173 (2006).
de Leeuw, A.M., McCarthy, S.P., Geerts, A. & Knook, D.L. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology 4, 392–403 (1984).
Friedman, S.L., Roll, F.J., Boyles, J. & Bissell, D.M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc. Natl. Acad. Sci. USA 82, 8681–8685 (1985).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Abe, M. et al. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216, 276–284 (1994).
Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Zhu, J. et al. β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129, 2891–2903 (2002).
Fässler, R. & Meyer, M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 9, 1896–1908 (1995).
Stephens, L.E. et al. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9, 1883–1895 (1995).
Abraham, S., Kogata, N., Fässler, R. & Adams, R.H. Integrin β1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ. Res. 102, 562–570 (2008).
Hinz, B. et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 180, 1340–1355 (2012).
Armulik, A., Genové, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Patsenker, E. et al. Pharmacological inhibition of integrin αvβ3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology 50, 1501–1511 (2009).
Ignotz, R.A. & Massagué, J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 261, 4337–4345 (1986).
Roberts, A.B. et al. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 83, 4167–4171 (1986).
Leask, A. & Abraham, D.J. TGF-β signaling and the fibrotic response. FASEB J. 18, 816–827 (2004).
Lacy-Hulbert, A. et al. Ulcerative colitis and autoimmunity induced by loss of myeloid αv integrins. Proc. Natl. Acad. Sci. USA 104, 15823–15828 (2007).
Proctor, J.M., Zang, K., Wang, D., Wang, R. & Reichardt, L.F. Vascular development of the brain requires β8 integrin expression in the neuroepithelium. J. Neurosci. 25, 9940–9948 (2005).
Hodivala-Dilke, K.M. et al. β3-integrin–deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103, 229–238 (1999).
Huang, X., Griffiths, M., Wu, J., Farese, R.V. Jr. & Sheppard, D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol. Cell. Biol. 20, 755–759 (2000).
Huang, X.Z. et al. Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J. Cell Biol. 133, 921–928 (1996).
Henderson, N.C. et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA 103, 5060–5065 (2006).
Henderson, N.C. et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 172, 288–298 (2008).
McCarty, J.H. et al. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132, 165–176 (2005).
Nagarajan, S.R. et al. R-isomers of Arg-Gly-Asp (RGD) mimics as potent αvβ3 inhibitors. Bioorg. Med. Chem. 15, 3783–3800 (2007).
Shannon, K.E. et al. Anti-metastatic properties of RGD-peptidomimetic agents S137 and S247. Clin. Exp. Metastasis 21, 129–138 (2004).
Acknowledgements
This work was supported by a Wellcome Trust Intermediate Clinical Fellowship (ref. 085187) to N.C.H., National Institutes of Health grants HL102292, HL53949 and AI077439 (to D.S.), a University of California, San Francisco (UCSF) Liver Center Tool and Technology grant (to N.C.H) and P30 DK026743 (UCSF Liver Center). We thank K. Thorn at the UCSF Nikon Imaging Center for assistance with image analysis. We also thank C. Her, N. Wu, S. Huling, D. Rodrigues and R. Aucott for expert technical assistance. We also acknowledge the contribution of M. Singh (chemical synthesis of compounds CWHM 12 and CWHM 96), D. Tajfirouz, S. Freeman and M. Yates at Saint Louis University for technical assistance in conducting integrin functional assays to characterize compound activities. L. Reichardt (UCSF) provided Itgb8flox/flox mice and R. Hynes (Massachusetts Institute of Technology) provided Itgb3−/− mice on 129/svJae background. S. Violette (Biogen Idec) provided antibody to αvβ6 (human/mouse chimeric 2A1), W. Stallcup (Sanford-Burnham Medical Research Institute) provided antibody to PDGFR-β and H. Yagita (Juntendo University) provided antibody to αv integrin (clone RMV-7).
Author information
Authors and Affiliations
Contributions
N.C.H. and D.S. conceived and designed the project. N.C.H. performed the experiments with assistance from T.D.A., Y.K., M.M.G., J.D.R. and A.P.; J.H.M. contributed reagents; P.G.R., D.W.G. and M.J.P. designed and synthesized the small molecule αv integrin inhibitor (CWHM 12) and performed the ligand-binding studies to characterize the in vitro potency of CWHM 12; J.J.M. and J.P.I. contributed reagents and provided substantial intellectual contribution; E.R. and C.B. contributed Pdgfrb-BAC-eGFP knock-in reporter mice; A.L.-H. contributed Itgavflox/flox mice; R.H.A. contributed Pdgfrb-Cre mice; N.C.H., T.D.A., Y.K., M.M.G. and D.S. analyzed data and N.C.H., J.P.I. and D.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.G.R. and D.W.G. hold equity in Antegrin Therapeutics, LLC.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Methods (PDF 4801 kb)
Rights and permissions
About this article
Cite this article
Henderson, N., Arnold, T., Katamura, Y. et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19, 1617–1624 (2013). https://doi.org/10.1038/nm.3282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3282
This article is cited by
-
Fibroblast and myofibroblast activation in normal tissue repair and fibrosis
Nature Reviews Molecular Cell Biology (2024)
-
NO-sensitive guanylyl cyclase discriminates pericyte-derived interstitial from intra-alveolar myofibroblasts in murine pulmonary fibrosis
Respiratory Research (2023)
-
Mechanical stiffness promotes skin fibrosis through Piezo1-mediated arginine and proline metabolism
Cell Death Discovery (2023)
-
Back to the future: targeting the extracellular matrix to treat systemic sclerosis
Nature Reviews Rheumatology (2023)
-
Hepatic inflammatory responses in liver fibrosis
Nature Reviews Gastroenterology & Hepatology (2023)