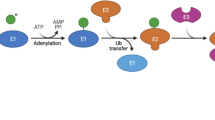
Key Points
-
Reversible post-translational protein modification by small ubiquitin-like modifier (SUMO), sumoylation, is a crucial mechanism in the maintenance of genomic integrity, in the regulation of proper gene expression patterns and in numerous signal transduction pathways and is thus essential for cell and tissue homeostasis in all eukaryotes.
-
Numerous protein complexes contain multiple sumoylated proteins, suggesting that sumoylation regulates not only the activity of individual substrates but that of entire functional complexes. Furthermore, in complex signalling pathways, sumoylation frequently targets multiple elements, in some cases antagonizing the activity of one element while promoting that of another within the same pathway.
-
Various biotic and abiotic stresses substantially alter the level of global cellular sumoylation. Importantly, numerous human tumours display marked upregulation of SUMO pathway components. This 'SUMOness' of cancers may indeed be required by tumour cells to maintain the robustness of compromised or otherwise easily misregulated gene expression programmes and signalling pathways. Sumoylation would therefore contribute substantially to cancer cell survival and proliferation in a potentially hostile microenvironment.
-
Although strong inhibition of global cellular sumoylation carries obvious risks for all cells, partial or temporary inhibition may be sufficient to expose certain tumour-specific vulnerabilities (for example, susceptibility to inducers of apoptosis and/or senescence) and therefore could provide a promising approach for future therapeutic intervention in some settings.
Abstract
Post-translational protein modification by small ubiquitin-like modifier (SUMO), termed sumoylation, is an important mechanism in cellular responses to stress and one that appears to be upregulated in many cancers. Here, we examine the role of sumoylation in tumorigenesis as a possibly necessary safeguard that protects the stability and functionality of otherwise easily misregulated gene expression programmes and signalling pathways of cancer cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Flotho, A. & Melchior, F. Sumoylation: a regulatory protein modification in health and disease. Annu. Rev. Biochem. 82, 357–385 (2013).
Beltrao, P., Bork, P., Krogan, N. J. & van Noort, V. Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 9, 714 (2013).
Minguez, P. et al. Deciphering a global network of functionally associated post-translational modifications. Mol. Syst. Biol. 8, 599 (2012).
Sternsdorf, T., Jensen, K. & Will, H. Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J. Cell Biol. 139, 1621–1634 (1997).
Müller, S., Matunis, M. J. & Dejean, A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 17, 61–70 (1998).
Matunis, M. J., Coutavas, E. & Blobel, G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135, 1457–1470 (1996).
Mahajan, R., Delphin, C., Guan, T., Gerace, L. & Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88, 97–107 (1997). References 6 and 7 are the first to show that SUMO is a ubiquitin-like protein modifier.
Zhong, S. et al. Role of SUMO-1-modified PML in nuclear body formation. Blood 95, 2748–2752 (2000).
Tatham, M. H. et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat. Cell Biol. 10, 538–546 (2008).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML-RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10, 547–555 (2008). First demonstrated in yeast, references 9 and 10 report the function of the homologous mammalian StUbL RNF4 in the degradation of PML and PML–RARα.
Zhu, J. et al. A sumoylation site in PML/RARA is essential for leukemic transformation. Cancer Cell 7, 143–153 (2005).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Stehmeier, P. & Müller, S. Regulation of p53 family members by the ubiquitin-like SUMO system. DNA Repair 8, 491–498 (2009).
Dünnebier, T. et al. Common variants in the UBC9 gene encoding the SUMO-conjugating enzyme are associated with breast tumor grade. Int. J. Cancer 125, 596–602 (2009).
Dünnebier, T. et al. Polymorphisms in the UBC9 and PIAS3 genes of the SUMO-conjugating system and breast cancer risk. Breast Cancer Res. Treat. 121, 185–194 (2010).
Synowiec, E. et al. Efficacy of DNA double-strand breaks repair in breast cancer is decreased in carriers of the variant allele of the UBC9 gene c.73G>A polymorphism. Mutat. Res. 694, 31–38 (2010).
Veltman, I. M. et al. Fusion of the SUMO/Sentrin-specific protease 1 gene SENP1 and the embryonic polarity-related mesoderm development gene MESDC2 in a patient with an infantile teratoma and a constitutional t(12;15)(q13;q25). Hum. Mol. Genet. 14, 1955–1963 (2005).
Tagawa, H. et al. Molecular cytogenetic analysis of the breakpoint region at 6q21-22 in T-cell lymphoma/leukemia cell lines. Genes Chromosomes Cancer 34, 175–185 (2002).
Yang, W. et al. Small ubiquitin-like modifier 1–3 conjugation is activated in human astrocytic brain tumors and is required for glioblastoma cell survival. Cancer Sci. 104, 70–77 (2013).
Bellail, A. C., Olson, J. J. & Hao, C. SUMO1 modification stabilizes CDK6 protein and drives the cell cycle and glioblastoma progression. Nat. Commun. 5, 4234 (2014).
Chen, S. F. et al. Ubc9 expression predicts chemoresistance in breast cancer. Chin. J. Cancer 30, 638–644 (2011).
Li, J. et al. Cbx4 governs HIF-1α to potentiate angiogenesis of hepatocellular carcinoma by its SUMO E3 ligase activity. Cancer Cell 25, 118–131 (2014).
Moschos, S. J. et al. SAGE and antibody array analysis of melanoma-infiltrated lymph nodes: identification of Ubc9 as an important molecule in advanced-stage melanomas. Oncogene 26, 4216–4225 (2007).
Shao, D. F. et al. High-level SAE2 promotes malignant phenotype and predicts outcome in gastric cancer. Am. J. Cancer Res. 5, 140–154 (2014).
Zhang, H., Kuai, X., Ji, Z., Li, Z. & Shi, R. Over-expression of small ubiquitin-related modifier-1 and sumoylated p53 in colon cancer. Cell Biochem. Biophys. 67, 1081–1087 (2013).
Xiang-Ming, Y. et al. SENP1 regulates cell migration and invasion in neuroblastoma. Biotechnol. Appl. Biochem. 63, 435–440 (2016).
Sun, Z. et al. Overexpression of SENP3 in oral squamous cell carcinoma and its association with differentiation. Oncol. Rep. 29, 1701–1706 (2013).
Ding, X. et al. Overexpression of SENP5 in oral squamous cell carcinoma and its association with differentiation. Oncol. Rep. 20, 1041–1045 (2008).
Jacques, C. et al. Two-step differential expression analysis reveals a new set of genes involved in thyroid oncocytic tumors. J. Clin. Endocrinol. Metab. 90, 2314–2320 (2005).
Brems-Eskildsen, A. S. et al. Prediction and diagnosis of bladder cancer recurrence based on urinary content of hTERT, SENP1, PPP1CA, and MCM5 transcripts. BMC Cancer 10, 646 (2010).
Han, Y. et al. SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress. J. Biol. Chem. 285, 12906–12915 (2010).
Bawa-Khalfe, T. & Yeh, E. T. SUMO losing balance: SUMO proteases disrupt SUMO homeostasis to facilitate cancer development and progression. Genes Cancer 1, 748–752 (2010).
Bawa-Khalfe, T., Cheng, J., Wang, Z. & Yeh, E. T. Induction of the SUMO-specific protease 1 transcription by the androgen receptor in prostate cancer cells. J. Biol. Chem. 282, 37341–37349 (2007).
Moschos, S. J. et al. Expression analysis of Ubc9, the single small ubiquitin-like modifier (SUMO) E2 conjugating enzyme, in normal and malignant tissues. Hum. Pathol. 41, 1286–1298 (2010).
Watts, F. Z. Starting and stopping SUMOylation. What regulates the regulator? Chromosoma 122, 451–463 (2013).
Amente, S., Lavadera, M. L., Palo, G. D. & Majello, B. SUMO-activating SAE1 transcription is positively regulated by Myc. Am. J. Cancer Res. 2, 330–334 (2012).
Hoellein, A. et al. Myc-induced SUMOylation is a therapeutic vulnerability for B-cell lymphoma. Blood 124, 2081–2090 (2014).
Comerford, K. M. et al. Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proc. Natl Acad. Sci. USA 100, 986–991 (2003).
Shao, R. et al. Increase of SUMO-1 expression in response to hypoxia: direct interaction with HIF-1α in adult mouse brain and heart in vivo. FEBS Lett. 569, 293–300 (2004).
Ying, S., Dünnebier, T., Si, J. & Hamann, U. Estrogen receptor alpha and nuclear factor Y coordinately regulate the transcription of the SUMO-conjugating gene UBC9 in MCF-7 breast cancer cells. PLoS ONE 8, e75695 (2013).
Wu, F., Zhu, S., Ding, Y., Beck, W. T. & Mo, Y. Y. MicroRNA-mediated regulation of Ubc9 expression in cancer cells. Clin. Cancer Res. 15, 1550–1557 (2009).
Zhao, Z. et al. microRNA-214-mediated UBC9 expression in glioma. BMB Rep. 45, 641–646 (2012).
Sahin, U. et al. Interferon controls SUMO availability via the Lin28 and let-7 axis to impede virus replication. Nat. Commun. 5, 4187 (2014).
Licciardello, M. P. et al. NOTCH1 activation in breast cancer confers sensitivity to inhibition of SUMOylation. Oncogene 34, 3780–3790 (2015).
Tomasi, M. L. et al. S-Adenosyl methionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in murine liver and human cancers. Hepatology 56, 982–993 (2012).
Su, Y. F., Yang, T., Huang, H., Liu, L. F. & Hwang, J. Phosphorylation of Ubc9 by Cdk1 enhances SUMOylation activity. PLoS ONE 7, e34250 (2012).
Hsieh, Y. L. et al. Ubc9 acetylation modulates distinct SUMO target modification and hypoxia response. EMBO J. 32, 791–804 (2013).
Knipscheer, P. et al. Ubc9 sumoylation regulates SUMO target discrimination. Mol. Cell 31, 371–382 (2008).
Klug, H. et al. Ubc9 sumoylation controls SUMO chain formation and meiotic synapsis in Saccharomyces cerevisiae. Mol. Cell 50, 625–636 (2013).
Truong, K., Lee, T. D. & Chen, Y. Small ubiquitin-like modifier (SUMO) modification of E1 Cys domain inhibits E1 Cys domain enzymatic activity. J. Biol. Chem. 287, 15154–15163 (2012).
Lee, M. H., Mabb, A. M., Gill, G. B., Yeh, E. T. & Miyamoto, S. NF-κB induction of the SUMO protease SENP2: a negative feedback loop to attenuate cell survival response to genotoxic stress. Mol. Cell 43, 180–191 (2011).
Pinto, M. P. et al. Heat shock induces a massive but differential inactivation of SUMO-specific proteases. Biochim. Biophys. Acta 1823, 1958–1966 (2012).
Guo, C. & Henley, J. M. Wrestling with stress: roles of protein SUMOylation and deSUMOylation in cell stress response. IUBMB Life 66, 71–77 (2014).
Golebiowski, F. et al. System-wide changes to SUMO modifications in response to heat shock. Sci. Signal. 2, ra24 (2009).
Saitoh, H. & Hinchey, J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 275, 6252–6258 (2000).
Seifert, A., Schofield, P., Barton, G. J. & Hay, R. T. Proteotoxic stress reprograms the chromatin landscape of SUMO modification. Sci. Signal. 8, rs7 (2015).
Sydorskyy, Y. et al. A novel mechanism for SUMO system control: regulated Ulp1 nucleolar sequestration. Mol. Cell. Biol. 30, 4452–4462 (2010).
Guo, C. et al. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J. 32, 1514–1528 (2013).
Bossis, G. & Melchior, F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21, 349–357 (2006).
Wang, Y., Yang, J. & Yi, J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid. Redox Signal. 16, 649–657 (2012).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Davalos, A. R., Coppe, J. P., Campisi, J. & Desprez, P. Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 29, 273–283 (2010).
Laberge, R. M., Awad, P., Campisi, J. & Desprez, P. Y. Epithelial–mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 5, 39–44 (2012).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Ohanna, M. et al. Senescent cells develop a PARP-1 and nuclear factor-κB-associated secretome (PNAS). Genes Dev. 25, 1245–1261 (2011).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Neyret-Kahn, H. et al. Sumoylation at chromatin governs coordinated repression of a transcriptional program essential for cell growth and proliferation. Genome Res. 23, 1563–1579 (2013). This study describes chromatin-associated sumoylation dynamics in cellular senescence.
Li, T. et al. Expression of SUMO-2/3 induced senescence through p53- and pRB-mediated pathways. J. Biol. Chem. 281, 36221–36227 (2006).
Bischof, O. et al. The E3 SUMO ligase PIASy is a regulator of cellular senescence and apoptosis. Mol. Cell 22, 783–794 (2006).
Yates, K. E., Korbel, G. A., Shtutman, M., Roninson, I. B. & DiMaio, D. Repression of the SUMO-specific protease Senp1 induces p53-dependent premature senescence in normal human fibroblasts. Aging Cell 7, 609–621 (2008).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Jackson, S. P. & Durocher, D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol. Cell 49, 795–807 (2013).
Dantuma, N. P. & van Attikum, H. Spatiotemporal regulation of posttranslational modifications in the DNA damage response. EMBO J. 35, 6–23 (2016).
Wan, J., Subramonian, D. & Zhang, X. D. SUMOylation in control of accurate chromosome segregation during mitosis. Curr. Protein Pept. Sci. 13, 467–481 (2012).
Rodriguez, A. & Pangas, S. A. Regulation of germ cell function by SUMOylation. Cell Tissue Res. 363, 47–55 (2016).
Cubenas-Potts, C. & Matunis, M. J. SUMO: a multifaceted modifier of chromatin structure and function. Dev. Cell 24, 1–12 (2013). An excellent review of SUMO function in all chromatin transactions.
Shayeghi, M., Doe, C. L., Tavassoli, M. & Watts, F. Z. Characterisation of Schizosaccharomyces pombe rad31, a UBA-related gene required for DNA damage tolerance. Nucleic Acids Res. 25, 1162–1169 (1997).
al-Khodairy, F., Enoch, T., Hagan, I. M. & Carr, A. M. The Schizosaccharomyces pombe hus5 gene encodes a ubiquitin conjugating enzyme required for normal mitosis. J. Cell Sci. 108, 475–486 (1995).
Galanty, Y. et al. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462, 935–939 (2009).
Morris, J. R. et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 462, 886–890 (2009).
Galanty, Y., Belotserkovskaya, R., Coates, J. & Jackson, S. P. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 26, 1179–1195 (2012). Using microscopy and in vivo laser microablation techniques, references 79–81 demonstrate the direct roles of sumoylation at DNA double-strand breaks through the recruitment of SUMO-targeted and SUMO-regulated ubiquitin ligases.
Bassi, C. et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341, 395–399 (2013).
Xhemalce, B., Seeler, J. S., Thon, G., Dejean, A. & Arcangioli, B. Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J. 23, 3844–3853 (2004).
Soustelle, C. et al. A new Saccharomyces cerevisiae strain with a mutant Smt3-deconjugating Ulp1 protein is affected in DNA replication and requires Srs2 and homologous recombination for its viability. Mol. Cell. Biol. 24, 5130–5143 (2004).
Branzei, D. et al. Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell 127, 509–522 (2006).
Shima, H. et al. Activation of the SUMO modification system is required for the accumulation of RAD51 at sites of DNA damage. J. Cell Sci. 126, 5284–5292 (2013).
Ouyang, K. J., Yagle, M. K., Matunis, M. J. & Ellis, N. A. BLM SUMOylation regulates ssDNA accumulation at stalled replication forks. Front. Genet. 4, 167 (2013).
Kessler, J. D. et al. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science 335, 348–353 (2012). Provides evidence from a synthetic lethal screen in RAS-driven breast cancer cells of the concept of non-oncogene addiction pertaining to sumoylation.
Yu, B. et al. Oncogenesis driven by the Ras/Raf pathway requires the SUMO E2 ligase Ubc9. Proc. Natl Acad. Sci. USA 112, E1724–E1733 (2015). Shows that growth of RAS-driven colorectal cancer cells require sumoylation of chromatin regulators such as KAP1, thus providing further evidence for the role of sumoylation in non-oncogene addiction.
Psakhye, I. & Jentsch, S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 151, 807–820 (2012). An elegant and thorough study demonstrating the importance of sumoylation in regulating the function of yeast double-strand DNA repair complex function.
Hendriks, I. A., Treffers, L. W., Verlaan-de Vries, M., Olsen, J. V. & Vertegaal, A. C. SUMO-2 orchestrates chromatin modifiers in response to DNA damage. Cell Rep. 10, 1778–1791 (2015).
Huang, T. T., Wuerzberger-Davis, S. M., Wu, Z. H. & Miyamoto, S. Sequential modification of NEMO/IKKγ by SUMO-1 and ubiquitin mediates NF-κB activation by genotoxic stress. Cell 115, 565–576 (2003). Provides the first demonstration that NEMO sumoylation is instrumental in mediating DNA damage-induced NF-κB activation.
Li, F. & Sethi, G. Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta 1805, 167–180 (2010).
Brach, M. A. et al. Ionizing radiation induces expression and binding activity of the nuclear factor κ B. J. Clin. Invest. 88, 691–695 (1991). First report demonstrating that genotoxic stress induces NF-κB activation.
Mabb, A. M., Wuerzberger-Davis, S. M. & Miyamoto, S. PIASy mediates NEMO sumoylation and NF-κB activation in response to genotoxic stress. Nat. Cell Biol. 8, 986–993 (2006).
Yang, Y. et al. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-κB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol. Cell. Biol. 31, 2774–2786 (2011).
Sabatel, H., Pirlot, C., Piette, J. & Habraken, Y. Importance of PIKKs in NF-κB activation by genotoxic stress. Biochem. Pharmacol. 82, 1371–1383 (2011).
McCool, K. W. & Miyamoto, S. DNA damage-dependent NF-κB activation: NEMO turns nuclear signaling inside out. Immunol. Rev. 246, 311–326 (2012).
Liu, X., Chen, W., Wang, Q., Li, L. & Wang, C. Negative regulation of TLR inflammatory signaling by the SUMO-deconjugating enzyme SENP6. PLoS Pathog. 9, e1003480 (2013).
Wuerzberger-Davis, S. M., Nakamura, Y., Seufzer, B. J. & Miyamoto, S. NF-κB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene 26, 641–651 (2006).
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D. & Paull, T. T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Stilmann, M. et al. A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IκB kinase activation. Mol. Cell 36, 365–378 (2009).
Piret, B. & Piette, J. Topoisomerase poisons activate the transcription factor NF-κB in ACH-2 and CEM cells. Nucleic Acids Res. 24, 4242–4248 (1996).
Renner, F., Moreno, R. & Schmitz, M. L. SUMOylation-dependent localization of IKKɛ in PML nuclear bodies is essential for protection against DNA-damage-triggered cell death. Mol. Cell 37, 503–515 (2010).
Longley, D. B. & Johnston, P. G. Molecular mechanisms of drug resistance. J. Pathol. 205, 275–292 (2005).
Bossis, G. et al. The ROS/SUMO axis contributes to the response of acute myeloid leukemia cells to chemotherapeutic drugs. Cell Rep. 7, 1815–1823 (2014). Provides evidence that low-ROS AML cells are chemoresistant because they fail to attenuate cellular sumoylation and that chemosensitivity in such cells can be restored by ROS or by inhibiting sumoylation.
Virchow, R. in Die Krankhaften Geschwülste Vol. 1 57–71 (Verlag von August Hirschwald, 1863) (in German).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Desterro, J. M., Rodriguez, M. S. & Hay, R. T. SUMO-1 modification of IκBα inhibits NF-κB activation. Mol. Cell 2, 233–239 (1998).
Aillet, F. et al. Heterologous SUMO-2/3-ubiquitin chains optimize IκBα degradation and NF-κB activity. PLoS ONE 7, e51672 (2012).
Kracklauer, M. P. & Schmidt, C. At the crossroads of SUMO and NF-κB. Mol. Cancer 2, 39 (2003).
Liu, B. et al. Negative regulation of NF-κB signaling by PIAS1. Mol. Cell. Biol. 25, 1113–1123 (2005).
Tahk, S. et al. Control of specificity and magnitude of NF-κB and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc. Natl Acad. Sci. USA 104, 11643–11648 (2007).
Liu, Y., Bridges, R., Wortham, A. & Kulesz-Martin, M. NF-κB repression by PIAS3 mediated RelA SUMOylation. PLoS ONE 7, e37636 (2012).
Liu, B. et al. Proinflammatory stimuli induce IKKα-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 129, 903–914 (2007).
Shuai, K. & Liu, B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat. Rev. Immunol. 5, 593–605 (2005).
Decque, A. et al. Sumoylation coordinates the repression of inflammatory and anti-viral gene-expression programs during innate sensing. Nat. Immunol. 17, 140–149 (2016). Demonstrates that in mouse immune cells, hyposumoylation induces a massive monomorphic inflammatory type I interferon response upon TLR engagement.
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Mani, S. A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Morrison, C. D., Parvani, J. G. & Schiemann, W. P. The relevance of the TGF-β paradox to EMT–MET programs. Cancer Lett. 341, 30–40 (2013).
Kang, J. S., Saunier, E. F., Akhurst, R. J. & Derynck, R. The type I TGF-β receptor is covalently modified and regulated by sumoylation. Nat. Cell Biol. 10, 654–664 (2008).
Imoto, S. et al. Regulation of transforming growth factor-β signaling by protein inhibitor of activated STAT, PIASy through Smad3. J. Biol. Chem. 278, 34253–34258 (2003).
Lin, X., Liang, M., Liang, Y. Y., Brunicardi, F. C. & Feng, X. H. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J. Biol. Chem. 278, 31043–31048 (2003).
Chang, C. C., Lin, D. Y., Fang, H. I., Chen, R. H. & Shih, H. M. Daxx mediates the small ubiquitin-like modifier-dependent transcriptional repression of Smad4. J. Biol. Chem. 280, 10164–10173 (2005).
Netherton, S. J. & Bonni, S. Suppression of TGFβ-induced epithelial–mesenchymal transition like phenotype by a PIAS1 regulated sumoylation pathway in NMuMG epithelial cells. PLoS ONE 5, e13971 (2010).
Long, J., Zuo, D. & Park, M. Pc2-mediated sumoylation of Smad-interacting protein 1 attenuates transcriptional repression of E-cadherin. J. Biol. Chem. 280, 35477–35489 (2005).
Taylor, K. M. & Labonne, C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev. Cell 9, 593–603 (2005).
Lee, P. C. et al. SUMOylated SoxE factors recruit Grg4 and function as transcriptional repressors in the neural crest. J. Cell Biol. 198, 799–813 (2012). A nice illustration of the importance of sumoylation in attenuating synergy between two transcription factors, MITF and SOX9.
Cronin, J. C. et al. Frequent mutations in the MITF pathway in melanoma. Pigment Cell Melanoma Res. 22, 435–444 (2009).
Bertolotto, C. et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 480, 94–98 (2011).
Yokoyama, S. et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature 480, 99–103 (2011). References 133 and 134 illustrate that loss of a sumoylation site in a transcription factor, MITF, represents a cancer predisposition in humans.
Cai, Q., Verma, S. C., Kumar, P., Ma, M. & Robertson, E. S. Hypoxia inactivates the VHL tumor suppressor through PIASy-mediated SUMO modification. PLoS ONE 5, e9720 (2010).
Sun, L. et al. PIASy mediates hypoxia-induced SIRT1 transcriptional repression and epithelial-to-mesenchymal transition in ovarian cancer cells. J. Cell Sci. 126, 3939–3947 (2013).
Carbia-Nagashima, A. et al. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1α during hypoxia. Cell 131, 309–323 (2007).
Xu, Y. et al. Induction of SENP1 in endothelial cells contributes to hypoxia-driven VEGF expression and angiogenesis. J. Biol. Chem. 285, 36682–36688 (2010).
Busca, R., Berra, E., Pouyssegur, J. & Ballotti, R. HIF1α est une nouvelle cible du facteur de transcription MITF Implication de la cascade AMPc-MITF-HIF1α dans le developpement des melanomes. Med. Sci. 22, 10–13 (2006) (in French).
Cai, Q. & Robertson, E. S. Ubiquitin/SUMO modification regulates VHL protein stability and nucleocytoplasmic localization. PLoS ONE 5, e12636 (2010).
Cheng, J., Kang, X., Zhang, S. & Yeh, E. T. SUMO-specific protease 1 is essential for stabilization of HIF1α during hypoxia. Cell 131, 584–595 (2007).
Huang, C. et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 28, 2748–2762 (2009).
Hart, Y. & Alon, U. The utility of paradoxical components in biological circuits. Mol. Cell 49, 213–221 (2013).
Kitano, H. Biological robustness. Nat. Rev. Genet. 5, 826–837 (2004). An excellent review of the design principles that ensure the stability of complex biological systems.
Borkent, M. et al. A serial shRNA screen for roadblocks to reprogramming identifies the protein modifier SUMO2. Stem Cell Reports 6, 704–716 (2016). Reports that SUMO2, but, interestingly, not SUMO1, represents a strong barrier to the production of induced pluripotent stem cells from differentiated fibroblasts.
Luo, J., Solimini, N. L. & Elledge, S. J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009).
Fukuda, I. et al. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem. Biol. 16, 133–140 (2009).
Fukuda, I. et al. Kerriamycin B inhibits protein SUMOylation. J. Antibiot. 62, 221–224 (2009).
Kim, Y. S., Nagy, K., Keyser, S. & Schneekloth, J. S. Jr. An electrophoretic mobility shift assay identifies a mechanistically unique inhibitor of protein sumoylation. Chem. Biol. 20, 604–613 (2013).
Hirohama, M. et al. Spectomycin B1 as a novel SUMOylation inhibitor that directly binds to SUMO E2. ACS Chem. Biol. 8, 2635–2642 (2013).
Kho, C. et al. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat. Commun. 6, 7229 (2015).
Kumar, A. & Zhang, K. Y. Advances in the development of SUMO specific protease (SENP) inhibitors. Comput. Struct. Biotechnol. J. 13, 204–211 (2015).
Hendriks, I. A. & Vertegaal, A. C. A comprehensive compilation of SUMO proteomics. Nat. Rev. Mol. Cell Biol. 17, 581–595 (2016). An indispensable reference summarizing all available human SUMO substrates and their modification sites discovered by proteomic approaches.
Eifler, K. & Vertegaal, A. C. SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem. Sci. 40, 779–793 (2015).
Peuscher, M. H. & Jacobs, J. J. Posttranslational control of telomere maintenance and the telomere damage response. Cell Cycle 11, 1524–1534 (2012).
Verger, A., Perdomo, J. & Crossley, M. Modification with SUMO. EMBO Rep. 4, 137–142 (2003).
Gill, G. Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr. Opin. Genet. Dev. 13, 108–113 (2003).
Stielow, B. et al. Identification of SUMO-dependent chromatin-associated transcriptional repression components by a genome-wide RNAi screen. Mol. Cell 29, 742–754 (2008).
Stielow, B., Sapetschnig, A., Wink, C., Kruger, I. & Suske, G. SUMO-modified Sp3 represses transcription by provoking local heterochromatic gene silencing. EMBO Rep. 9, 899–906 (2008).
Paakinaho, V., Kaikkonen, S., Makkonen, H., Benes, V. & Palvimo, J. J. SUMOylation regulates the chromatin occupancy and anti-proliferative gene programs of glucocorticoid receptor. Nucleic Acids Res. 42, 1575–1592 (2013).
Liu, B., Tahk, S., Yee, K. M., Fan, G. & Shuai, K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science 330, 521–525 (2010).
Maison, C. et al. SUMOylation promotes de novo targeting of HP1α to pericentric heterochromatin. Nat. Genet. 43, 220–227 (2011). Shows a role for the sumoylation of HP1α in the de novo establishment of heterochromatin through an RNA binding mechanism.
Bawa-Khalfe, T. et al. Differential expression of SUMO-specific protease 7 variants regulates epithelial–mesenchymal transition. Proc. Natl Acad. Sci. USA 109, 17466–17471 (2012).
Poleshko, A. et al. Human factors and pathways essential for mediating epigenetic gene silencing. Epigenetics 9, 1280–1289 (2014).
Ling, Y. et al. Modification of de novo DNA methyltransferase 3a (Dnmt3a) by SUMO-1 modulates its interaction with histone deacetylases (HDACs) and its capacity to repress transcription. Nucleic Acids Res. 32, 598–610 (2004).
Gomez-del Arco, P., Koipally, J. & Georgopoulos, K. Ikaros SUMOylation: switching out of repression. Mol. Cell. Biol. 25, 2688–2697 (2005).
Kotaja, N., Karvonen, U., Janne, O. A. & Palvimo, J. J. The nuclear receptor interaction domain of GRIP1 is modulated by covalent attachment of SUMO-1. J. Biol. Chem. 277, 30283–30288 (2002).
Liu, H. W. et al. Chromatin modification by SUMO-1 stimulates the promoters of translation machinery genes. Nucleic Acids Res. 40, 10172–10186 (2012).
Chang, P. C. et al. The chromatin modification by SUMO-2/3 but not SUMO-1 prevents the epigenetic activation of key immune-related genes during Kaposi's sarcoma associated herpesvirus reactivation. BMC Genomics 14, 824 (2013).
Cheng, C. Y. et al. An improved ChIP–seq peak detection system for simultaneously identifying post-translational modified transcription factors by combinatorial fusion, using SUMOylation as an example. BMC Genomics 15 (Suppl. 1), S1 (2014).
Niskanen, E. A. et al. Global SUMOylation on active chromatin is an acute heat stress response restricting transcription. Genome Biol. 16, 153 (2015). Illustrates the dynamics of SUMO2 and SUMO3 chromatin occupancy under heat stress.
Srikumar, T. et al. Global analysis of SUMO chain function reveals multiple roles in chromatin regulation. J. Cell Biol. 201, 145–163 (2013).
Mukherjee, S., Cruz-Rodriguez, O., Bolton, E. & Iniguez-Lluhi, J. A. The in vivo role of androgen receptor SUMOylation as revealed by androgen insensitivity syndrome and prostate cancer mutations targeting the proline/glycine residues of synergy control motifs. J. Biol. Chem. 287, 31195–31206 (2012).
Tempe, D. et al. SUMOylation of the inducible (c-Fos:c-Jun)/AP-1 transcription complex occurs on target promoters to limit transcriptional activation. Oncogene 33, 921–927 (2014).
Rosonina, E., Duncan, S. M. & Manley, J. L. SUMO functions in constitutive transcription and during activation of inducible genes in yeast. Genes Dev. 24, 1242–1252 (2010).
Hickey, C. M., Wilson, N. R. & Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 13, 755–766 (2012).
Ikeuchi, Y. et al. TIF1γ protein regulates epithelial–mesenchymal transition by operating as a small ubiquitin-like modifier (SUMO) E3 ligase for the transcriptional regulator SnoN1. J. Biol. Chem. 289, 25067–25078 (2014).
Fattet, L. et al. TIF1γ requires sumoylation to exert its repressive activity on TGFβ signaling. J. Cell Sci. 126, 3713–3723 (2013).
Agricola, E., Randall, R. A., Gaarenstroom, T., Dupont, S. & Hill, C. S. Recruitment of TIF1γ to chromatin via its PHD finger-bromodomain activates its ubiquitin ligase and transcriptional repressor activities. Mol. Cell 43, 85–96 (2011).
Lee, P. S., Chang, C., Liu, D. & Derynck, R. Sumoylation of Smad4, the common Smad mediator of transforming growth factor-β family signaling. J. Biol. Chem. 278, 27853–27863 (2003).
Semenza, G. L. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 33, 207–214 (2012).
Carter, S., Bischof, O., Dejean, A. & Vousden, K. H. C-Terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat. Cell Biol. 9, 428–435 (2007).
Wu, S. Y. & Chiang, C. M. Crosstalk between sumoylation and acetylation regulates p53-dependent chromatin transcription and DNA binding. EMBO J. 28, 1246–1259 (2009).
Ledl, A., Schmidt, D. & Müller, S. Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene 24, 3810–3818 (2005).
Huang, J. et al. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat. Commun. 3, 911 (2012).
Gonzalez-Prieto, R., Cuijpers, S. A., Kumar, R., Hendriks, I. A. & Vertegaal, A. C. c-Myc is targeted to the proteasome for degradation in a SUMOylation-dependent manner, regulated by PIAS1, SENP7 and RNF4. Cell Cycle 14, 1859–1872 (2015).
Ding, B., Sun, Y. & Huang, J. Overexpression of SKI oncoprotein leads to p53 degradation through regulation of MDM2 protein sumoylation. J. Biol. Chem. 287, 14621–14630 (2012).
Lee, M. H. et al. SUMO-specific protease SUSP4 positively regulates p53 by promoting Mdm2 self-ubiquitination. Nat. Cell Biol. 8, 1424–1431 (2006).
Huang, H. J. et al. β-Catenin SUMOylation is involved in the dysregulated proliferation of myeloma cells. Am. J. Cancer Res. 5, 309–320 (2015).
Kim, J. H. et al. Roles of sumoylation of a reptin chromatin-remodelling complex in cancer metastasis. Nat. Cell Biol. 8, 631–639 (2006).
Wang, X. D. et al. SUMO-modified nuclear cyclin D1 bypasses Ras-induced senescence. Cell Death Differ. 18, 304–314 (2011).
Tan, M. Y. et al. SUMO-specific protease 2 suppresses cell migration and invasion through inhibiting the expression of MMP13 in bladder cancer cells. Cell. Physiol. Biochem. 32, 542–548 (2013).
Liu, B. et al. PIAS1 regulates breast tumorigenesis through selective epigenetic gene silencing. PLoS ONE 9, e89464 (2014).
Cashman, R., Cohen, H., Ben-Hamo, R., Zilberberg, A. & Efroni, S. SENP5 mediates breast cancer invasion via a TGFβRI SUMOylation cascade. Oncotarget 5, 1071–1082 (2014).
Coppola, D., Parikh, V., Boulware, D. & Blanck, G. Substantially reduced expression of PIAS1 is associated with colon cancer development. J. Cancer Res. Clin. Oncol. 135, 1287–1291 (2009).
Wei, J. et al. mRNA expression of BRCA1, PIAS1, and PIAS4 and survival after second-line docetaxel in advanced gastric cancer. J. Natl Cancer Inst. 103, 1552–1556 (2011).
Song, J. G. et al. SUMO-specific protease 6 promotes gastric cancer cell growth via deSUMOylation of FoxM1. Tumour Biol. 36, 9865–9871 (2015).
Ren, Y. H. et al. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial–mesenchymal transition in gastric cancer cells. Oncotarget 5, 7093–7104 (2014).
McDoniels-Silvers, A. L., Nimri, C. F., Stoner, G. D., Lubet, R. A. & You, M. Differential gene expression in human lung adenocarcinomas and squamous cell carcinomas. Clin. Cancer Res. 8, 1127–1138 (2002).
Inamura, K. et al. A metastatic signature in entire lung adenocarcinomas irrespective of morphological heterogeneity. Hum. Pathol. 38, 702–709 (2007).
Rabellino, A. et al. The SUMO E3-ligase PIAS1 regulates the tumor suppressor PML and its oncogenic counterpart PML-RARA. Cancer Res. 72, 2275–2284 (2012).
Liu, X. et al. Knockdown of SUMO-activating enzyme subunit 2 (SAE2) suppresses cancer malignancy and enhances chemotherapy sensitivity in small cell lung cancer. J. Hematol. Oncol. 8, 67 (2015).
Driscoll, J. J. et al. The sumoylation pathway is dysregulated in multiple myeloma and is associated with adverse patient outcome. Blood 115, 2827–2834 (2010).
Xu, J. et al. SENP1 inhibition induces apoptosis and growth arrest of multiple myeloma cells through modulation of NF-κB signaling. Biochem. Biophys. Res. Commun. 460, 409–415 (2015).
Chien, W. et al. PIAS4 is an activator of hypoxia signalling via VHL suppression during growth of pancreatic cancer cells. Br. J. Cancer 109, 1795–1804 (2013).
Ma, C. et al. SUMO-specific protease 1 regulates pancreatic cancer cell proliferation and invasion by targeting MMP-9. Tumour Biol. 35, 12729–12735 (2014).
Li, P. et al. Heterogeneous expression and functions of androgen receptor co-factors in primary prostate cancer. Am. J. Pathol. 161, 1467–1474 (2002).
Hoefer, J. et al. PIAS1 is increased in human prostate cancer and enhances proliferation through inhibition of p21. Am. J. Pathol. 180, 2097–2107 (2012).
Li, T., Huang, S., Dong, M., Gui, Y. & Wu, D. Prognostic impact of SUMO-specific protease 1 (SENP1) in prostate cancer patients undergoing radical prostatectomy. Urol. Oncol. 31, 1539–1545 (2013).
Wang, L. & Banerjee, S. Differential PIAS3 expression in human malignancy. Oncol. Rep. 11, 1319–1324 (2004).
Acknowledgements
The authors apologize to the researchers whose contributions were not cited here owing to space constraints. The authors gratefully acknowledge the reviewers for their constructive criticisms and G. Fourel, J. Campbell, A. Krebs and the members of the Dejean Lab for valuable input. Work in the authors' laboratory is supported by grants from the European Research Council (ERC AdG-294341 'SUMOSTRESS'), the Institute National du Cancer (INCa), the Ligue Nationale Contre le Cancer (LNCC, Equipe Labellisée) and Odyssey-RE (to A.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- SUMO
-
Small protein belonging to the UBL (ubiquitin-like) family of proteins that are subject to enzyme-mediated covalent attachment to other proteins or chemical groups. Three functional paralogues exist in mammals, SUMO1, SUMO2 and SUMO3, of which the latter two (often called SUMO2/3) can form poly-SUMO chains in vivo.
- Acute promyelocytic leukaemia
-
(APL). Leukaemia characterized by chromosomal translocations producing oncogenic fusion proteins of the retinoic acid receptor-α (RARα) and promyelocytic leukaemia protein (PML; most cases), PLZF, NPM or NUMA (rare cases); associated with a differentiation block producing immature granulocytes (promyelocytes).
- PML nuclear bodies
-
Approximately one-micron-sized mammalian subnuclear structures containing sumoylated PML and SP100 as principal components, as well as numerous other, more transient proteins often implicated in regulating cellular stress responses such as genotoxic and oxidative stress.
- SUMO-targeted ubiquitin ligase
-
(StUbL). A ubiquitin E3 ligase containing SUMO-interacting motifs that binds SUMO-modified proteins to promote their degradation by the ubiquitin–proteasome system.
- SUMO E2 conjugating enzyme
-
The enzyme (usually called UBC9) that transfers SUMO to its substrate. In contrast to the ubiquitin E2, the SUMO E2 enzyme is unique in all organisms.
- SUMO1/sentrin specific peptidase
-
(SENP). SUMO-specific cysteine protease capable of removing mature SUMO from its substrate (isopeptidase activity) and/or from its carboxy-terminal extension (maturation endopeptidase activity). Mammals possess six SENPs (SENP1, SENP2, SENP3, SENP5, SENP6 and SENP7), as well as the unrelated DESI1, DESI2 and USPL1 SUMO proteases.
- SUMO E1 activating enzyme
-
In the SUMO system, the ATP-requiring, dimeric SAE1–SAE2 (also known as AOS1–UBA2) enzyme that makes the first high-energy thioester bond with mature SUMO that is necessary for the subsequent transfer steps.
- SUMO E3 ligase
-
The protein that brings the SUMO-charged E2 enzyme and substrate together. The canonical E3 ligases in mammals are the PIAS proteins (PIAS1–4) and MMS21 (also known as NSE2).
- Negatively charged amino acid-dependent sumoylation motif
-
A variant of the sumoylation consensus motif, ψKxE/D, (consisting of a bulky hydrophobic residue, ψ, the modified lysine, K, any amino acid, X, and an acidic residue, E or D) that contains additional negatively charged (for example, E or D) or phosphorylated (S, T or Y) residues further downstream.
- SUMO-interacting motif
-
A short peptide motif facilitating non-covalent interactions (generally weak) with SUMO. SUMO-targeted ubiquitin ligases usually contain several such motifs resulting in stronger interactions with poly- or multi-sumoylated substrates.
- Senescence-associated heterochromatin foci
-
Microscopically visible, dense nuclear structures with features of heterochromatin (for example, repressive histone marks) that are characteristic of some senescent cells.
- ChIP–seq
-
Chromatin immunoprecipitation and next-generation sequencing; a method used to determine genome-wide binding patterns of chromatin-associated proteins using specific antibodies.
- SUMO-regulated ubiquitin ligases
-
(SrUbLs). Directly sumoylated ubiquitin E3 ligases, such as RNF8, RNF168 or BRCA1, involved in DNA double-strand break repair.
- Synthetic lethal interactions
-
Occur when inhibition or mutation of one or the other of two given factors is viable but simultaneous inhibition is lethal. Non-oncogene addiction is considered a specific example of this concept.
- Pathogen-associated molecular patterns
-
(PAMPs). Pathogen-derived peptide, cell wall (for example, lipopolysaccharide) or nucleic acid fragments recognized by specific pattern recognition receptors (PRRs) that induce the innate immune response.
- Toll-like receptor
-
(TLR). Member of a family of pattern recognition receptors that bind pathogen-associated molecular patterns.
- Warburg effect
-
The metabolic shift, seen in many tumours, towards aerobic glycolysis that favours high anabolic rates and is compatible with, and inducible by, hypoxic conditions.
- Modules
-
Systems theory concept describing organization of functional elements, for example, in physical structures or metabolic pathways; usually viewed as contributing to the robustness of the entire system.
- Non-oncogene addiction
-
The concept that targeting certain stress tolerance or other non-oncogene pathways inhibits or kills cancer cells.
Rights and permissions
About this article
Cite this article
Seeler, JS., Dejean, A. SUMO and the robustness of cancer. Nat Rev Cancer 17, 184–197 (2017). https://doi.org/10.1038/nrc.2016.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.143
This article is cited by
-
ROS-mediated up-regulation of SAE1 by Helicobacter pylori promotes human gastric tumor genesis and progression
Journal of Translational Medicine (2024)
-
Stabilization of Pin1 by USP34 promotes Ubc9 isomerization and protein sumoylation in glioma stem cells
Nature Communications (2024)
-
SUMOylation modulates eIF5A activities in both yeast and pancreatic ductal adenocarcinoma cells
Cellular & Molecular Biology Letters (2024)
-
Epigenetic modification of m6A regulator proteins in cancer
Molecular Cancer (2023)
-
ACE2 in chronic disease and COVID-19: gene regulation and post-translational modification
Journal of Biomedical Science (2023)