Key Points
-
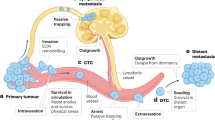
Metastasis progression can be viewed as a stepwise sequence of events, which is mediated by different classes of metastasis genes.
-
For each type of cancer, the clinical course of these events occurs with distinct temporal kinetics and in unique organ sites.
-
The long latency period of certain tumour types suggests the further evolution or 'speciation' of malignant cells in the microenvironments of a particular organ. The acquisition of pro-metastatic functions earlier during primary tumour formation might enable other cancer subtypes to relapse more quickly.
-
The organ specificity of metastatic cells is determined by unique infiltrative and colonization functions required after their dissemination from a primary tumour.
-
New insights into the importance of latency and organ-specific colonization should be considered in the design of optimized therapeutic strategies.
Abstract
Metastasis to distant organs is an ominous feature of most malignant tumours but the natural history of this process varies in different cancers. The cellular origin, intrinsic properties of the tumour, tissue affinities and circulation patterns determine not only the sites of tumour spread, but also the temporal course and severity of metastasis to vital organs. Striking disparities in the natural progression of different cancers raise important questions about the evolution of metastatic traits, the genetic determinants of these properties and the mechanisms that lead to the selection of metastatic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Christofori, G. New signals from the invasive front. Nature 441, 444–450 (2006).
Gupta, G. P. & Massague, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Steeg, P. S. Tumor metastasis: mechanistic insights and clinical challenges. Nature Med. 12, 895–904 (2006).
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Rev. Cancer 3, 453–458 (2003).
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Yin, J. J. et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Minn, A. J. et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Invest. 115, 44–55 (2005). This paper shows that pleural effusion-derived metastatic cell populations are heterogeneous in their ability to colonize different organs, supporting the notion that various target organs impose different requirements on arriving tumour cells.
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003). This paper shows that in vivo selection enriches for bone metastatic ability and identifies genetic mediators of bone metastatic colonization.
Edlund, M., Sung, S. Y. & Chung, L. W. Modulation of prostate cancer growth in bone microenvironments. J. Cell. Biochem. 91, 686–705 (2004).
Triozzi, P. L., Eng, C. & Singh, A. D. Targeted therapy for uveal melanoma. Cancer Treat. Rev. 34, 247–258 (2008).
Hess, K. R. et al. Metastatic patterns in adenocarcinoma. Cancer 106, 1624–1633 (2006). A recent clinical study that reports the frequency of organ-specific relapse in 11 different types of adenocarcinomas from over 4,000 patients.
Patanaphan, V., Salazar, O. M. & Risco, R. Breast cancer: metastatic patterns and their prognosis. South. Med. J. 81, 1109–1112 (1988).
Schmidt-Kittler, O. et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc. Natl Acad. Sci. USA 100, 7737–7742 (2003). This paper shows that disseminated tumour cells have different and fewer aberrations than their matched primary tumours, suggesting that dissemination is an early event during cancer development.
Karrison, T. G., Ferguson, D. J. & Meier, P. Dormancy of mammary carcinoma after mastectomy. J. Natl Cancer Inst. 91, 80–85 (1999).
Feld, R., Rubinstein, L. V. & Weisenberger, T. H. Sites of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J. Clin. Oncol. 2, 1352–1358 (1984).
Hoffman, P. C., Mauer, A. M. & Vokes, E. E. Lung cancer. Lancet 355, 479–485 (2000).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Rev. Cancer 2, 563–572 (2002).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Moody, S. E. et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2, 451–461 (2002).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Minna, J. D., Kurie, J. M. & Jacks, T. A big step in the study of small cell lung cancer. Cancer Cell 4, 163–166 (2003).
Klein, C. A. The systemic progression of human cancer: a focus on the individual disseminated cancer cell—the unit of selection. Adv. Cancer Res. 89, 35–67 (2003).
Chiang, A. C. & Massagué, J. Molecular basis of metastasis. N. Engl. J. Med. 359, 2814–2823 (2008).
Nguyen, D. X. & Massagué, J. Genetic determinants of cancer metastasis. Nature Rev. Genet. 8, 341–352 (2007).
Yang, J. & Weinberg, R. A. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Hu, G. et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell 15, 9–20 (2009). A study that mechanistically links the pro-metastatic gene metadherin with resistance to chemotherapy.
Stein, U. et al. MACC1, a newly identified key regulator of HGF–MET signaling, predicts colon cancer metastasis. Nature Med. 15, 59–67 (2009).
Guo, C. et al. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer 47, 939–946 (2008).
Tavazoie, S. F. et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 (2008).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Rev. Cancer 2, 584–593 (2002).
Lee, Y. T. Patterns of metastasis and natural courses of breast carcinoma. Cancer Metastasis Rev. 4, 153–172 (1985).
Johansson, J. E. et al. Natural history of early, localized prostate cancer. JAMA 291, 2713–2719 (2004).
Nieto, J., Grossbard, M. L. & Kozuch, P. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist 13, 562–576 (2008).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Vogelstein, B. et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 (1988).
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004).
Baker, S. J. et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 244, 217–221 (1989).
Markowitz, S. et al. Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science 268, 1336–1338 (1995).
Jones, S. et al. Comparative lesion sequencing provides insights into tumor evolution. Proc. Natl Acad. Sci. USA 105, 4283–4288 (2008). A study that evaluated the frequency and timing of somatic mutations to estimate the clinical course of colorectal metastatic progression.
Kedrin, D., van Rheenen, J., Hernandez, L., Condeelis, J. & Segall, J. E. Cell motility and cytoskeletal regulation in invasion and metastasis. J. Mammary Gland Biol. Neoplasia 12, 143–152 (2007).
Weber, G. F. Molecular mechanisms of metastasis. Cancer Lett. 270, 181–190 (2008).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Ewald, A. J., Brenot, A., Duong, M., Chan, B. S. & Werb, Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570–581 (2008).
Kouros-Mehr, H. et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13, 141–152 (2008).
Podsypanina, K. et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science 321, 1841–1844 (2008). This paper showed that phenotypically normal mouse mammary cells introduced into the mouse circulation can infiltrate the lungs and survive, leading to tumour initiation.
Ince, T. A. et al. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell 12, 160–170 (2007). This study showed that there are intrinsic differences in the tumorigenic and metastatic capabilities of different mammary cell types.
Mani, S. A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Bandyopadhyay, S. et al. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nature Med. 12, 933–938 (2006).
Karpatkin, S. & Pearlstein, E. Role of platelets in tumor cell metastases. Ann. Intern. Med. 95, 636–641 (1981).
Nieswandt, B., Hafner, M., Echtenacher, B. & Mannel, D. N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 59, 1295–1300 (1999).
Im, J. H. et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 64, 8613–8619 (2004).
Jain, S. et al. Platelet glycoprotein Ibα supports experimental lung metastasis. Proc. Natl Acad. Sci. USA 104, 9024–9028 (2007).
Paku, S., Dome, B., Toth, R. & Timar, J. Organ-specificity of the extravasation process: an ultrastructural study. Clin. Exp. Metastasis 18, 481–492 (2000).
Lalor, P. F., Lai, W. K., Curbishley, S. M., Shetty, S. & Adams, D. H. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J. Gastroenterol. 12, 5429–5439 (2006).
Schluter, K. et al. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am. J. Pathol. 169, 1064–1073 (2006).
Brown, D. M. & Ruoslahti, E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 5, 365–374 (2004). Using phage-display libraries — a technology that had previously allowed this group to identify tissue-specific vasculature differences (or 'zipcodes') — this paper identifies metadherin as a lung-specific homing molecule.
Kopp, H. G., Avecilla, S. T., Hooper, A. T. & Rafii, S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology 20, 349–356 (2005).
Weis, S., Cui, J., Barnes, L. & Cheresh, D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 167, 223–229 (2004).
Gupta, G. P. et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446, 765–770 (2007).
Karnoub, A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563 (2007).
Padua, D. et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008). References 62 and 63 exemplify how paracrine signals from the stroma of a primary tumour can stimulate departing cancer cells to extravasate into the lung without affecting primary tumorigenesis.
Wang, H. et al. Tumor cell α3β1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis. J. Cell Biol. 164, 935–941 (2004).
Weil, R. J., Palmieri, D. C., Bronder, J. L., Stark, A. M. & Steeg, P. S. Breast cancer metastasis to the central nervous system. Am. J. Pathol. 167, 913–920 (2005).
Minn, A. J. et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc. Natl Acad. Sci. USA 104, 6740–6745 (2007).
Smid, M. et al. Genes associated with breast cancer metastatic to bone. J. Clin. Oncol. 24, 2261–2267 (2006).
Nesbitt, J. C., Putnam, J. B. Jr, Walsh, G. L., Roth, J. A. & Mountain, C. F. Survival in early-stage non-small cell lung cancer. Ann. Thorac. Surg. 60, 466–472 (1995).
Ries, L. A. G. et al. SEER Cancer Statistics Review, 1975–2005 National Cancer Institute [online] http://seer.cancer.gov/csr/1975_2005/index.html (2008)
Janne, P. A. et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 95, 1528–1538 (2002).
Briele, H. A. & Das Gupta, T. K. Natural history of cutaneous malignant melanoma. World J. Surg. 3, 255–270 (1979).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869–10874 (2001).
Garraway, L. A. et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122 (2005).
Gupta, P. B. et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nature Genet. 37, 1047–1054 (2005).
Wong, D. J. et al. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2, 333–344 (2008). This study shows an association between the expression of embryonic stem cell gene modules in primary tumours and increased metastatic potential.
Varambally, S. et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002).
Kleer, C. G. et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc. Natl Acad. Sci. USA 100, 11606–11611 (2003).
Varambally, S. et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322, 1695–1699 (2008).
Gregory, P. A. et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biol. 10, 593–601 (2008).
Ma, L., Teruya-Feldstein, J. & Weinberg, R. A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682–688 (2007).
Demicheli, R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin. Cancer Biol. 11, 297–306 (2001).
Braun, S. et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N. Engl. J. Med. 342, 525–533 (2000).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008). This paper shows that dissemination of tumour cells can occur at early stages of primary tumour development in ERBB2 and PyMT mouse models. Moreover, transplantation of pre-malignant DTCs into recipient bone marrow releases these cells from dormancy.
White, D. E. et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170 (2004).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERKMAPK activity as a determinant of tumor growth and dormancy; regulation by p38SAPK. Cancer Res. 63, 1684–1695 (2003).
Aguirre-Ghiso, J. A., Ossowski, L. & Rosenbaum, S. K. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 64, 7336–7345 (2004).
Nash, K. T. et al. Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J. Natl Cancer Inst. 99, 309–321 (2007).
Xu, L., Begum, S., Hearn, J. D. & Hynes, R. O. GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc. Natl Acad. Sci. USA 103, 9023–9028 (2006).
Park, Y. G. et al. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nature Genet. 37, 1055–1062 (2005).
Holmgren, L., O'Reilly, M. S. & Folkman, J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Med. 1, 149–153 (1995).
Almog, N. et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 69, 836–844 (2009).
Naumov, G. N., Akslen, L. A. & Folkman, J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5, 1779–1787 (2006).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Becker, S., Becker-Pergola, G., Wallwiener, D., Solomayer, E. F. & Fehm, T. Detection of cytokeratin-positive cells in the bone marrow of breast cancer patients undergoing adjuvant therapy. Breast Cancer Res. Treat. 97, 91–96 (2006).
Ling, L. J. et al. A novel mouse model of human breast cancer stem-like cells with high CD44+CD24−/lower phenotype metastasis to human bone. Chin. Med. J. 121, 1980–1986 (2008).
Gupta, G. P. et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc. Natl Acad. Sci. USA 104, 19506–19511 (2007).
Swarbrick, A., Roy, E., Allen, T. & Bishop, J. M. Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response. Proc. Natl Acad. Sci. USA 105, 5402–5407 (2008).
Park, B. K. et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nature Med. 13, 62–69 (2007).
Fitzgerald, D. P. et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metastasis 25, 799–810 (2008).
Sharma, S. V., Bell, D. W., Settleman, J. & Haber, D. A. Epidermal growth factor receptor mutations in lung cancer. Nature Rev. Cancer 7, 169–181 (2007).
Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007).
Bean, J. et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl Acad. Sci. USA 104, 20932–20937 (2007).
Aragon-Ching, J. B. & Zujewski, J. A. CNS metastasis: an old problem in a new guise. Clin. Cancer Res. 13, 1644–1647 (2007).
Fidler, I. J., Yano, S., Zhang, R. D., Fujimaki, T. & Bucana, C. D. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 3, 53–57 (2002).
Wiedswang, G. et al. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin. Cancer Res. 10, 5342–5348 (2004).
Erler, J. T. et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 (2006).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009). References 106 and 107 describe the metastasis progression gene LOX and how it can be induced in the primary tumour to mediate one function at the primary site and a different function in secondary organs, both of which are required for metastasis.
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Hiratsuka, S. et al. The S100A8–serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nature Cell Biol. 10, 1349–1355 (2008).
Condeelis, J. & Pollard, J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 (2006).
Kim, S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 (2009).
Bierie, B. & Moses, H. L. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nature Rev. Cancer 6, 506–520 (2006).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Wikman, H., Vessella, R. & Pantel, K. Cancer micrometastasis and tumour dormancy. APMIS 116, 754–770 (2008).
Pantel, K. & Brakenhoff, R. H. Dissecting the metastatic cascade. Nature Rev. Cancer 4, 448–456 (2004).
Klein, C. A. et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet 360, 683–689 (2002).
Schardt, J. A. et al. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell 8, 227–239 (2005).
Ramaswamy, S., Ross, K. N., Lander, E. S. & Golub, T. R. A molecular signature of metastasis in primary solid tumors. Nature Genet. 33, 49–54 (2003).
Weigelt, B. et al. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc. Natl Acad. Sci. USA 100, 15901–15905 (2003).
Acknowledgements
We thank members of the Massagué lab for insightful discussions. The primary research for the topic of this review is supported by grants from the National Institutes of Health, the Hearst Foundation and the Kleberg Foundation. D.X.N was a postdoctoral fellow of the Damon Runyon Cancer Research Foundation. J.M. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Infiltration
-
The entry of cancer cells into distant organs through invasion and extravasation.
- Colonization
-
The outgrowth of metastatic cells that have co-opted a distant organ microenvironment.
- Latency
-
The time between primary tumour diagnosis and clinically detectable metastatic outgrowths.
- Intravasation
-
The entry of tumour cells into the bloodstream.
- Extravasation
-
The exit of tumour cells from capillary beds into the parenchyma of an organ.
- Basal breast cancer
-
A more aggressive subtype of breast cancer with characteristics of mammary basal cells, and that typically lacks oestrogen and progesterone receptors.
- Luminal breast cancer
-
A subtype of breast cancer with characteristics of cells that originate from the normal lumen or ducts of the mammary gland.
- Dormancy
-
A state of cellular quiescence in the G0 phase of the cell cycle. When referring to a tumour cell mass, dormancy describes a balanced state of proliferation and apoptosis.
- Angiogenic switch
-
The transition between a non-angiogenic state of the tumour cell mass and a neovascularized state that enables tumour oxygenation and growth.
- Tumour-propagating phenotype
-
The ability of the infiltrated tumour cells to reinitiate growth at the secondary site. This is referred to by some investigators as the 'cancer stem cell phenotype'.
- Metastatic speciation
-
An evolutionary process by which new metastatic populations arise, owing to the various selective pressures that act on the heterogeneous cancer cells escaping the primary tumour.
- Gliosis
-
Stimulation of astrocytes in injured areas of the brain.
Rights and permissions
About this article
Cite this article
Nguyen, D., Bos, P. & Massagué, J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9, 274–284 (2009). https://doi.org/10.1038/nrc2622
Issue Date:
DOI: https://doi.org/10.1038/nrc2622
This article is cited by
-
The role of the desmosomal protein desmocollin 2 in tumour progression in triple negative breast cancer patients
Cancer Cell International (2023)
-
MUC16 promotes triple-negative breast cancer lung metastasis by modulating RNA-binding protein ELAVL1/HUR
Breast Cancer Research (2023)
-
Hypoxia-induced PLOD2 promotes clear cell renal cell carcinoma progression via modulating EGFR-dependent AKT pathway activation
Cell Death & Disease (2023)
-
Biology, vulnerabilities and clinical applications of circulating tumour cells
Nature Reviews Cancer (2023)
-
Small extracellular vesicles delivering lncRNA WAC-AS1 aggravate renal allograft ischemia‒reperfusion injury by inducing ferroptosis propagation
Cell Death & Differentiation (2023)