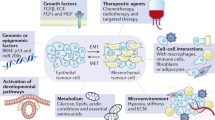
Abstract
Metastasis is the leading cause of cancer-related deaths, but it is unclear how cancer cells escape their primary sites in epithelia and disseminate to other sites in the body. One emerging possibility is that transformed epithelial cells could invade the underlying tissue by a process called cell extrusion, which epithelia use to remove cells without disrupting their barrier function. Typically, during normal cell turnover, live cells extrude apically from the epithelium into the lumen and later die by anoikis; however, several oncogenic mutations shift cell extrusion basally, towards the tissue that the epithelium encases. Tumour cells with high levels of survival and motility signals could use basal extrusion to escape from the tissue and migrate to other sites within the body.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012).
Rosenblatt, J., Raff, M. C. & Cramer, L. P. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847–1857 (2001).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Kiehart, D. P., Galbraith, C. G., Edwards, K. A., Rickoll, W. L. & Montague, R. A. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471–490 (2000).
Marinari, E. et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545 (2012).
Madara, J. L. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J. Membr. Biol. 116, 177–184 (1990).
Guan, Y. et al. Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 300, C1404–C1414 (2011).
Gu, Y., Forostyan, T., Sabbadini, R. & Rosenblatt, J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J. Cell Biol. 193, 667–676 (2011).
Kuipers, D. et al. Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J. Cell Sci. 127, 1229–1241 (2014).
Andrade, D. & Rosenblatt, J. Apoptotic regulation of epithelial cellular extrusion. Apoptosis 16, 491–501 (2011).
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010).
Slattum, G., McGee, K. M. & Rosenblatt, J. P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J. Cell Biol. 186, 693–702 (2009).
Hogan, C. et al. Characterization of the interface between normal and transformed epithelial cells. Nature Cell Biol. 11, 460–467 (2009).
Kajita, M. et al. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J. Cell Sci. 123, 171–180 (2010).
Donker, M. et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized Phase III trial. J. Clin. Oncol. 31, 4054–4059 (2013).
Bleyer, A. & Welch, H. G. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 367, 1998–2005 (2012).
Hartenstein, V., Younossi-Hartenstein, A. & Lekven, A. Delamination and division in the Drosophila neurectoderm: spatiotemporal pattern, cytoskeletal dynamics, and common control by neurogenic and segment polarity genes. Dev. Biol. 165, 480–499 (1994).
Kelley, L. C., Lohmer, L. L., Hagedorn, E. J. & Sherwood, D. R. Traversing the basement membrane in vivo: a diversity of strategies. J. Cell Biol. 204, 291–302 (2014).
Wyckoff, J. B., Pinner, S. E., Gschmeissner, S., Condeelis, J. S. & Sahai, E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523 (2006).
Friedl, P., Locker, J., Sahai, E. & Segall, J. E. Classifying collective cancer cell invasion. Nature Cell Biol. 14, 777–783 (2012).
Marshall, T. W., Lloyd, I. E., Delalande, J. M., Nathke, I. & Rosenblatt, J. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol. Biol. Cell 22, 3962–3970 (2011).
Slattum, G., Gu, Y., Sabbadini, R. & Rosenblatt, J. Autophagy in oncogenic K-Ras promotes basal extrusion of epithelial cells by degrading S1P. Curr. Biol. 24, 19–28 (2014).
Clevers, H. Wnt breakers in colon cancer. Cancer Cell 5, 5–6 (2004).
Oving, I. M. & Clevers, H. C. Molecular causes of colon cancer. Eur. J. Clin. Invest. 32, 448–457 (2002).
van Es, J. H., Giles, R. H. & Clevers, H. C. The many faces of the tumor suppressor gene APC. Exp. Cell Res. 264, 126–134 (2001).
Kita, K., Wittmann, T., Nathke, I. S. & Waterman-Storer, C. M. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol. Biol. Cell 17, 2331–2345 (2006).
Mogensen, M. M., Tucker, J. B., Mackie, J. B., Prescott, A. R. & Nathke, I. S. The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells. J. Cell Biol. 157, 1041–1048 (2002).
Zumbrunn, J., Kinoshita, K., Hyman, A. A. & Nathke, I. S. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 β phosphorylation. Curr. Biol. 11, 44–49 (2001).
Minde, D. P., Anvarian, Z., Rudiger, S. G. & Maurice, M. M. Messing up disorder: how do missense mutations in the tumor suppressor protein APC lead to cancer? Mol. Cancer 10, 101 (2011).
Schepers, A. & Clevers, H. Wnt signaling, stem cells, and cancer of the gastrointestinal tract. Cold Spring Harb. Perspect. Biol. 4, a007989 (2012).
Neuzillet, C., Hammel, P., Tijeras-Raballand, A., Couvelard, A. & Raymond, E. Targeting the Ras-ERK pathway in pancreatic adenocarcinoma. Cancer Metastasis Rev. 32, 147–162 (2013).
Aviel-Ronen, S., Blackhall, F. H., Shepherd, F. A. & Tsao, M. S. K-ras mutations in non-small-cell lung carcinoma: a review. Clin. Lung Cancer 8, 30–38 (2006).
Jiang, Y., Kimchi, E. T., Staveley-O'Carroll, K. F., Cheng, H. & Ajani, J. A. Assessment of K-ras mutation: a step toward personalized medicine for patients with colorectal cancer. Cancer 115, 3609–3617 (2009).
Liu, J. S., Farlow, J. T., Paulson, A. K., Labarge, M. A. & Gartner, Z. J. Programmed cell-to-cell variability in Ras activity triggers emergent behaviors during mammary epithelial morphogenesis. Cell Rep. 2, 1461–1470 (2012).
White, E. Exploiting the bad eating habits of Ras-driven cancers. Genes Dev. 27, 2065–2071 (2013).
Mathew, R. & White, E. Autophagy, stress, and cancer metabolism: what doesn't kill you makes you stronger. Cold Spring Harb. Symp. Quant. Biol. 76, 389–396 (2011).
Mancias, J. D. & Kimmelman, A. C. Targeting autophagy addiction in cancer. Oncotarget 2, 1302–1306 (2011).
Cufi, S. et al. The anti-malarial chloroquine overcomes primary resistance and restores sensitivity to trastuzumab in HER2-positive breast cancer. Sci. Rep. 3, 2469 (2013).
Rosenfeldt, M. T. et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 504, 296–300 (2013).
Royer, C. & Lu, X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death Differ. 18, 1470–1477 (2011).
Macara, I. G. & McCaffrey, L. Cell polarity in morphogenesis and metastasis. Phil. Trans. R. Soc. B 368, 20130012 (2013).
Gildea, J. J. et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 62, 6418–6423 (2002).
Struckhoff, A. P., Rana, M. K. & Worthylake, R. A. RhoA can lead the way in tumor cell invasion and metastasis. Front. Biosci. 16, 1915–1926 (2011).
Plodinec, M. et al. The nanomechanical signature of breast cancer. Nature Nanotechnol. 7, 757–765 (2012).
Lee, M. H. et al. Mismatch in mechanical and adhesive properties induces pulsating cancer cell migration in epithelial monolayer. Biophys. J. 102, 2731–2741 (2012).
Yang, J. & Weinberg, R. A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 15, 87–96 (2005).
Yilmaz, M., Christofori, G. & Lehembre, F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol. Med. 13, 535–541 (2007).
Kissa, K. & Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Chambers, K. F. et al. Stroma regulates increased epithelial lateral cell adhesion in 3D culture: a role for actin/cadherin dynamics. PLoS ONE 6, e18796 (2011).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869–10874 (2001).
Perez-Enciso, M. & Tenenhaus, M. Prediction of clinical outcome with microarray data: a partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 112, 581–592 (2003).
Livasy, C. A. et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod. Pathol. 19, 264–271 (2006).
Prat, A., Ellis, M. J. & Perou, C. M. Practical implications of gene-expression-based assays for breast oncologists. Nature Rev. Clin. Oncol. 9, 48–57 (2012).
Zomer, A. et al. Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells 31, 602–606 (2013).
Wang, W. et al. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 67, 3505–3511 (2007).
Nakasone, E. S. et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21, 488–503 (2012).
Condeelis, J. & Weissleder, R. In vivo imaging in cancer. Cold Spring Harb. Perspect. Biol. 2, a003848 (2010).
Roussos, E. T. et al. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle-T transgenic mouse mammary tumors. Breast Cancer Res. 12, R101 (2010).
Boimel, P. J. et al. Contribution of CXCL12 secretion to invasion of breast cancer cells. Breast Cancer Res. 14, R23 (2012).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771 (2013).
White, R., Rose, K. & Zon, L. Zebrafish cancer: the state of the art and the path forward. Nature Rev. Cancer 13, 624–636 (2013).
Jung, D.-W. et al. A novel zebrafish human tumor xenograft model validated for anti-cancer drug screening. Mol. BioSyst. 8, 1930–1939 (2012).
Konantz, M. et al. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann. NY Acad. Sci. 1266, 124–137 (2012).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Nieto, M. A. The early steps of neural crest development. Mech. Dev. 105, 27–35 (2001).
Wang, W. et al. Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 15, 138–145 (2005).
Acknowledgements
The authors thank the US National Institutes of Health (NIH) for an Innovator Award DP2OD002056-01 and an RO1 1R01GM102169-01 to J.R., and for an NIH Developmental Biology Training Grant 5T32 HDO7491 to G.M.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Slattum, G., Rosenblatt, J. Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nat Rev Cancer 14, 495–501 (2014). https://doi.org/10.1038/nrc3767
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3767
This article is cited by
-
IDO-1 inhibitor INCB24360 elicits distant metastasis of basal extruded cancer cells in pancreatic ductal adenocarcinoma
Acta Pharmacologica Sinica (2023)
-
The ECM and tissue architecture are major determinants of early invasion mediated by E-cadherin dysfunction
Communications Biology (2023)
-
Cell adhesion molecule BVES functions as a suppressor of tumor cells extrusion in hepatocellular carcinoma metastasis
Cell Communication and Signaling (2022)
-
Sequential Ras/MAPK and PI3K/AKT/mTOR pathways recruitment drives basal extrusion in the prostate-like gland of Drosophila
Nature Communications (2020)
-
Electrochemiluminescence Sensor for Cancer Cell Detection Based on H2O2-Triggered Stimulus Response System
Journal of Analysis and Testing (2020)