Key Points
-
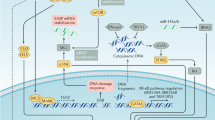
Induction of senescence, a stable state of cell cycle arrest, was originally described in normal cells, but it can also be induced in tumour cells in response to various stresses.
-
Senescent cells are metabolically active. In contrast to tumour cells, which typically preferentially use glycolysis in the presence of oxygen to generate energy, senescent cells can exhibit hyperactive mitochondrial respiration (oxidative phosphorylation) in some contexts.
-
Autophagy is activated during senescence, but its importance varies depending on the context.
-
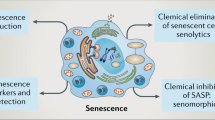
The senescence-associated secretory phenotype (SASP) mediates the diverse functionality of senescent cells in an autocrine and paracrine manner, including reinforcing or inducing senescence, activating an immune response and even promoting tumorigenesis, depending on the context.
-
Senescent cells can be eliminated through a SASP-induced immune response, which can involve both innate and adaptive immunity.
-
Various triggers, such as tissue damage or tumorigenesis-associated stresses, can cause stromal cell senescence, which may either facilitate or inhibit tumour progression, depending on the context.
Abstract
The core aspect of the senescent phenotype is a stable state of cell cycle arrest. However, this is a disguise that conceals a highly active metabolic cell state with diverse functionality. Both the cell-autonomous and the non-cell-autonomous activities of senescent cells create spatiotemporally dynamic and context-dependent tissue reactions. For example, the senescence-associated secretory phenotype (SASP) provokes not only tumour-suppressive but also tumour-promoting responses. Senescence is now increasingly considered to be an integrated and widespread component that is potentially important for tumour development, tumour suppression and the response to therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Campisi, J. & D'adda Di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nature Rev. Mol. Cell. Biol. 8, 729–740 (2007).
D'adda Di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nature Rev. Cancer 8, 512–522 (2008).
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Salama, R., Sadaie, M., Hoare, M. & Narita, M. Cellular senescence and its effector programs. Genes Dev. 28, 99–114 (2014).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Hoare, M. & Narita, M. Transmitting senescence to the cell neighbourhood. Nature Cell Biol. 15, 887–889 (2013).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
D'adda Di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Herbig, U., Jobling, W. A., Chen, B. P. C., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, 53, and p21(CIP1), but not p16(INK4a). Mol. Cell 14, 501–513 (2004).
Blasco, M. A. Mice with bad ends: mouse models for the study of telomeres and telomerase in cancer and aging. EMBO J. 24, 1095–1103 (2005).
González-Suárez, E., Samper, E., Flores, J. M. & Blasco, M. A. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nature Genet. 26, 114–117 (2000).
Greenberg, R. A. et al. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell 97, 515–525 (1999).
Qi, L. et al. Short telomeres and ataxia-telangiectasia mutated deficiency cooperatively increase telomere dysfunction and suppress tumorigenesis. Cancer Res. 63, 8188–8196 (2003).
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007).
Feldser, D. M. & Greider, C. W. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell 11, 461–469 (2007).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997). This seminal paper established the concept of OIS.
Lin, A. W. et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12, 3008–3019 (1998).
Zhu, J., Woods, D., McMahon, M. & Bishop, J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12, 2997–3007 (1998).
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Collado, M. et al. Tumour biology: Senescence in premalignant tumours. Nature 436, 642–642 (2005).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005). This is a seminal study that identified OIS of human melanocytic naevi.
Lazzerini Denchi, E., Attwooll, C., Pasini, D. & Helin, K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol. Cell. Biol. 25, 2660–2672 (2005).
Shamma, A. et al. Rb regulates DNA damage response and cellular senescence through E2F-dependent suppression of N-ras isoprenylation. Cancer Cell 15, 255–269 (2009).
Courtois-Cox, S. et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell 10, 459–472 (2006).
Gewinner, C. et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 16, 115–125 (2009).
Chang, B. D. et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 59, 3761–3767 (1999).
Poele te, R. H., Okorokov, A. L., Jardine, L., Cummings, J. & Joel, S. P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62, 1876–1883 (2002).
Schmitt, C. A. et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 (2002). This was the first study showing that chemotherapy-induced tumour senescence contributed to improved survival in mice, in an apoptosis defective context.
Wu, C.-H. et al. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc. Natl Acad. Sci. USA 104, 13028–13033 (2007).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007). This was the first study to show that the innate immune response eliminates the senescent cells induced in tumours in mice.
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Reimann, M. et al. Tumor stroma-derived TGF-b limits Myc-Driven lymphomagenesisvia Suv39h1-dependent senescence. Cancer Cell 17, 262–272 (2010).
Ewald, J. A., Desotelle, J. A., Wilding, G. & Jarrard, D. F. Therapy-induced senescence in cancer. J. Natl Cancer Inst. 102, 1536–1546 (2010).
Nardella, C., Clohessy, J. G., Alimonti, A. & Pandolfi, P. P. Pro-senescence therapy for cancer treatment. Nature Rev. Cancer 11, 503–511 (2011).
Acosta, J. C. & Gil, J. Senescence: a new weapon for cancertherapy. Trends Cell Biol. 22, 211–219 (2012).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg's contributions to current concepts of cancer metabolism. Nature Rev. Cancer 11, 325–337 (2011).
Kaplon, J. et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112 (2013). This study established the metabolic profile of OIS cells, identifying active PDH with increased mitochondrial respiration during OIS.
Kondoh, H. et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 65, 177–185 (2005).
Dörr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013). This study shows that in a TIS model, the SASP, which provokes endoplasmic reticulum stress, is associated with increased glucose use, hyperactive mitochondrial respiration, and autophagy activation, and that TIS cells are sensitive to blocking glucose use or autophagy, which causes endoplasmic reticulum-related apoptosis.
Poulikakos, P. I. & Rosen, N. Mutant BRAF melanomas—dependence and resistance. Cancer Cell 19, 11–15 (2011).
Bonnet, S. et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11, 37–51 (2007).
Michelakis, E. D., Webster, L. & Mackey, J. R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 99, 989–994 (2008).
Haq, R. et al. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell 23, 302–315 (2013).
Vazquez, F. et al. PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 23, 287–301 (2013).
St-Pierre, J. et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127, 397–408 (2006).
Jiang, P., Du, W., Mancuso, A., Wellen, K. E. & Yang, X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493, 689–693 (2013). This study shows that malic enzymes and p53 reciprocally repress each other and that depletion of ME1 or ME2 induces senescence in both normal and tumour cells.
Baggetto, L. G. Deviant energetic metabolism of glycolytic cancer cells. Biochimie 74, 959–974 (1992).
Ren, J.-G., Seth, P., Everett, P., Clish, C. B. & Sukhatme, V. P. Induction of erythroid differentiation in human erythroleukemia cells by depletion of malic enzyme 2. PLoS ONE 5, e12520 (2010).
Quijano, C. et al. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle 11, 1383–1392 (2012).
Passos, J. F., Saretzki, G. & Zglinicki Von, T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 35, 7505–7513 (2007).
Passos, J. F. et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. Plos Biol. 5, e110 (2007).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Moiseeva, O. et al. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol. Cell. Biol. 29, 4495–4507 (2009).
Narita, M. et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011).
Young, A. R. J. et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 (2009). This study shows that autophagy is activated during senescence and that the inhibition of autophagy delays the onset of senescence and the SASP.
Korolchuk, V. I. et al. Lysosomal positioning coordinates cellular nutrient responses. Nature 13, 453–460 (2011).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011).
Wang, Y. et al. Autophagic activity dictates the cellular response to oncogenic RAS. Proc. Natl Acad. Sci. 109, 13325–13330 (2012).
Tuveson, D. A. et al. Endogenous oncogenic K-rasG12D stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Hoare, M., Young, A. R. J. & Narita, M. Autophagy in cancer: Having your cake and eating it. Semin. Cancer Biol. 21, 397–404 (2011).
Yang, Z. J., Chee, C. E., Huang, S. & Sinicrope, F. A. The role of autophagy in cancer: therapeutic implications. Mol. Cancer Ther. 10, 1533–1541 (2011).
Gewirtz, D. A. Autophagy and senescence in cancer therapy. J. Cell. Physiol. 229, 6–9 (2014).
Amaravadi, R. K. et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest. 117, 326–336 (2007).
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003).
Yue, Z., Jin, S., Yang, C., Levine, A. J. & Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl Acad. Sci. USA 100, 15077–15082 (2003).
Mariño, G. et al. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J. Biol. Chem. 282, 18573–18583 (2007).
Takamura, A. et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 25, 795–800 (2011).
Gewirtz, D. A. Autophagy and senescence: a partnership in search of definition. Autophagy 9, 808–812 (2013).
Lee, B. Y. et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187–195 (2006).
Gerland, L.-M. et al. Association of increased autophagic inclusions labeled for β-galactosidase with fibroblastic aging. Exp. Gerontol. 38, 887–895 (2003).
Leidal, A. M., Cyr, D. P., Hill, R. J., Lee, P. W. K. & McCormick, C. Subversion of autophagy by Kaposi's sarcoma-associated herpesvirus impairs oncogene-induced senescence. Cell Host Microbe 11, 167–180 (2012).
Mosieniak, G. et al. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech. Ageing Dev. 133, 444–455 (2012).
Goehe, R. W. et al. The autophagy-senescence connection in chemotherapy: must tumor cells (self) eat before they sleep? J. Pharmacol. Exp. Ther. 343, 763–778 (2012).
Knizhnik, A. V. et al. Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS ONE 8, e55665 (2013).
Patel, K. R. et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 5, 205ra133 (2013).
Qi, M. et al. Pseudolaric acid B-induced autophagy contributes to senescence via enhancement of ROS generation and mitochondrial dysfunction in murine fibrosarcoma L929 cells. J. Pharmacol. Sci. 121, 200–211 (2013).
Qi, M. et al. mTOR inactivation by ROS-JNK-p53 pathway plays an essential role in Psedolaric acid B induced autophagy-dependent senescence in murine fibrosarcoma L929 cells. Eur. J. Pharmacol. 715, 76–88 (2013).
Patschan, S. et al. Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 294, H1119–H1129 (2008).
Kang, H. T., Lee, K. B., Kim, S. Y., Choi, H. R. & Park, S. C. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS ONE 6, e23367 (2011).
Janku, F., McConkey, D. J., Hong, D. S. & Kurzrock, R. Autophagy as a target for anticancer therapy. Nature Rev. Clin. Oncol. 8, 528–539 (2011).
Amaravadi, R. K. et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 17, 654–666 (2011).
Rosenfeldt, M. T. et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature 504, 296–300 (2014).
Guo, J. Y. et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 27, 1447–1461 (2013).
Kuilman, T. & Peeper, D. S. Senescence-messaging secretome: SMS-ing cellular stress. Nature Rev. Cancer 9, 81–94 (2009).
Coppé, J.-P., Desprez, P.-Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008). References 91 and 92 describe the crucial role of pro-inflammatory cytokines and their receptors in senescence.
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biol. 15, 978–990 (2013). This study identified the inflammasome as a critical regulator of the SASP.
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Chien, Y. et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136 (2011).
Rodier, F. et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature 11, 973–979 (2009).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013). This is an interesting study that shows a functional connection between obesity-induced senescence and a stromal SASP.
Orjalo, A. V., Bhaumik, D., Gengler, B. K., Scott, G. K. & Campisi, J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl Acad. Sci. USA 106, 17031–17036 (2009).
Davalos, A. R. et al. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 201, 613–629 (2013).
Millis, A. J., Hoyle, M., McCue, H. M. & Martini, H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp. Cell Res. 201, 373–379 (1992).
Goldstein, S., Moerman, E. J., Fujii, S. & Sobel, B. E. Overexpression of plasminogen activator inhibitor type-1 in senescent fibroblasts from normal subjects and those with Werner syndrome. J. Cell. Physiol. 161, 571–579 (1994).
Kortlever, R. M., Higgins, P. J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature Cell Biol. 8, 877–884 (2006).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Parrinello, S., Coppé, J.-P., Krtolica, A. & Campisi, J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 118, 485–496 (2005).
Coppé, J.-P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. Plos Biol. 6, 2853–2868 (2008).
Hubackova, S. et al. IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine 'bystander senescence'. Aging 4, 932–951 (2012).
Wajapeyee, N., Serra, R. W., Zhu, X., Mahalingam, M. & Green, M. R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132, 363–374 (2008).
Scurr, L. L. et al. IGFBP7 is not required for B-RAF-induced melanocyte senescence. Cell 141, 717–727 (2010).
Wajapeyee, N., Serra, R. W., Zhu, X., Mahalingam, M. & Green, M. R. Role for IGFBP7 in senescence induction by BRAF. Cell 141, 746–747 (2010).
Jackson, J. G. et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell 21, 793–806 (2012).
Hoenicke, L. & Zender, L. Immune surveillance of senescent cells—biological significance in cancer- and non-cancer pathologies. Carcinogenesis 33, 1123–1126 (2012).
Kang, T.-W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011). This is a seminal study that identifies senescence surveillance involving the adaptive immune response.
Iannello, A., Thompson, T. W., Ardolino, M., Lowe, S. W. & Raulet, D. H. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J. Exp. Med. 210, 2057–2069 (2013). This study suggests that NK cells that are recruited to the tumour lesion by the SASP of senescent tumour cells may also kill non-senescent tumour cells.
Raulet, D. H., Gasser, S., Gowen, B. G., Deng, W. & Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 31, 413–441 (2013).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Rakhra, K. et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 18, 485–498 (2010).
Willimsky, G. & Blankenstein, T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 437, 141–146 (2005).
Guerra, C. et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19, 728–739 (2011).
Bavik, C. et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 66, 794–802 (2006).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008). This was the first study to show that HSC senescence controls fibrosis through the SASP.
Friedman, S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008).
Kim, K.-H., Chen, C.-C., Monzon, R. I. & Lau, L. F. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol. Cell. Biol. 33, 2078–2090 (2013).
Jun, J.-I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nature Cell Biol. 12, 676–685 (2010).
Burd, C. E. et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 152, 340–351 (2013). This study shows a close and general correlation between stromal senescence and early neoplastic events in a new p16-'monitor' mouse model.
Yang, G. et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl Acad. Sci. USA 103, 16472–16477 (2006).
Braumüller, H. et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 494, 361–365 (2013). This study shows that tumours can be induced into senescence by cytokines that are derived from TAA-activated CD4+ T H 1 cells.
Bergers, G., Javaherian, K., Lo, K. M., Folkman, J. & Hanahan, D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 284, 808–812 (1999).
Müller-Hermelink, N. et al. TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13, 507–518 (2008).
Casanovas, O., Hager, J. H., Chun, M. G. H. & Hanahan, D. Incomplete inhibition of the Rb tumor suppressor pathway in the context of inactivated p53 is sufficient for pancreatic islet tumorigenesis. Oncogene 24, 6597–6604 (2005).
Quesnel, B. Tumor dormancy and immunoescape. APMIS 116, 685–694 (2008).
Lengagne, R. et al. Distinct role for CD8 T cells toward cutaneous tumors and visceral metastases. J. Immunol. 180, 130–137 (2008).
Eyles, J. et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039 (2010).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature Rev. Cancer 13, 759–771 (2013).
Nickoloff, B. J., Ben-Neriah, Y. & Pikarsky, E. Inflammation and cancer: is the link as simple as we think? J. Invest. Dermatol. 124, x–xiv (2005).
Nickoloff, B. J. Creation of psoriatic plaques: the ultimate tumor suppressor pathway. A new model for an ancient T-cell-mediated skin disease. J. Cutan. Pathol. 28, 57–64 (2001).
Nickoloff, B. J. & Nestle, F. O. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113, 1664–1675 (2004).
Kaelin, W. G. & McKnight, S. L. Influence of metabolism on epigenetics and disease. Cell 153, 56–69 (2013).
Lu, C. & Thompson, C. B. Metabolic regulation of epigenetics. Cell. Metab. 16, 9–17 (2012).
Draoui, N. & Feron, O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis. Model. Mech. 4, 727–732 (2011).
Giancotti, F. G. Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013).
Martins, C. P., Brown-Swigart, L. & Evan, G. I. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127, 1323–1334 (2006).
Dickins, R. A. et al. Tissue-specific and reversible RNA interference in transgenic mice. Nature Genet. 39, 914–921 (2007).
Pribluda, A. et al. A senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell 24, 242–256 (2013).
Cristofalo, V. J., Lorenzini, A., Allen, R. G., Torres, C. & Tresini, M. Replicative senescence: a critical review. Mech. Ageing Dev. 125, 827–848 (2004).
Sharpless, N. E. & Depinho, R. A. How stem cells age and why this makes us grow old. Nature Rev. Mol. Cell Biol. 8, 703–713 (2007).
Baker, D. J. et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nature 10, 825–836 (2008).
Sousa-Victor, P. et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321 (2014).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2012).
Muñoz-Espín, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Chuprin, A. et al. Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev. 27, 2356–2366 (2013).
Alimonti, A. et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 120, 681–693 (2010).
Schlomm, T. et al. Clinical significance of p53 alterations in surgically treated prostate cancers. Mod. Pathol. 21, 1371–1378 (2008).
Song, M. S., Salmena, L. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor. Nature Rev. Mol. Cell. Biol. 13, 283–296 (2012).
Soucek, L. et al. Modelling Myc inhibition as a cancer therapy. Nature 455, 679–683 (2008).
Lin, H.-K. et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 464, 374–379 (2010).
Campaner, S. et al. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nature 12, 54–59 (2010).
Puyol, M. et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18, 63–73 (2010).
Chan, C.-H. et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell 154, 556–568 (2013).
Wall, M. et al. The mTORC1 inhibitor everolimus prevents and treats Eμ-Myc lymphoma by restoring oncogene-induced senescence. Cancer Discov. 3, 82–95 (2013).
Acknowledgements
The authors thank C. Frezza and D. T. Fearon for their thoughtful discussions, as well as M. Hoare, Masako Narita and other members of Narita group, for critical reading and discussions. This work was supported by the University of Cambridge, Cancer Research UK, Hutchison Whampoa and the Human Frontier Science Program (M.N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Warburg effect
-
A shift in glucose metabolism from mitochondrial oxidative phosphorylation to glycolysis in the presence of ample oxygen.
- NADPH
-
The reduced form of NADP+ and a source of reducing equivalents. Major sources of NADPH include the pentose phosphate pathway, NADP-dependent malic enzymes and NADP-dependent isocitrate dehydrogenase.
- Pancreatic intraepithelial neoplasias
-
(PanINs). Neoplasias that are usually induced by activating mutations in KRAS and represent the most common precursor lesion of pancreatic ductal adenocarcinoma.
- Cytokines
-
A broad class of small proteins that comprise chemokines, interferons, interleukins, lymphokines and tumour necrosis factors that are produced by various cells.
- Inflammasomes
-
Cytosolic multiprotein innate immune complexes that assemble in response to various stimuli and that activate the pro-inflammatory serine protease caspase 1. Inflammasomes initiate innate immune responses by cleaving the inflammatory cytokines pro-interleukin-1 ß (pro-IL-1ß) and pro-IL-18, leading to their activation and secretion.
- Macrophages
-
Generally, M1-polarized macrophages inhibit cell proliferation and cause tissue damage, whereas M2-polarized macrophages promote cell proliferation and tissue repair. The M1 and M2 macrophages promote the T helper 1 (TH1) and TH2 cell responses, respectively.
- Natural killer cells
-
(NK cells). A subgroup of white blood cells that is implicated in the killing of tumour or virus-infected cells. They are generally considered to be the cytotoxic lymphocytes of innate immunity owing to their lack of antigen-specific cell surface receptors, but they can also exert antibody-dependent cell cytotoxicity.
- TH1 cell
-
T helper 1 (TH1) cells are a subset of CD4+ T helper cells that secrete cytokines, including interferon-γ (IFNγ) and tumour necrosis factor (TNF). They are canonically associated with the stimulation of CD8+ cytotoxic T lymphocytes and macrophages, as well as antitumour immunity.
- Enterohepatic circulation
-
The circulation between the liver and the intestine. Bile acids and other substances excreted from the liver are reabsorbed by the enterocytes into the hepatic portal vein.
Rights and permissions
About this article
Cite this article
Pérez-Mancera, P., Young, A. & Narita, M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer 14, 547–558 (2014). https://doi.org/10.1038/nrc3773
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3773
This article is cited by
-
MicroRNAs-associated with FOXO3 in cellular senescence and other stress responses
Biogerontology (2024)
-
Bovine oviductal organoids: a multi-omics approach to capture the cellular and extracellular molecular response of the oviduct to heat stress
BMC Genomics (2023)
-
Cellular senescence triggers intracellular acidification and lysosomal pH alkalinized via ATP6AP2 attenuation in breast cancer cells
Communications Biology (2023)
-
Unraveling the potential of senescence-related genes in guiding clinical therapy of lung adenocarcinoma patients
Functional & Integrative Genomics (2023)
-
Could senescence phenotypes strike the balance to promote tumor dormancy?
Cancer and Metastasis Reviews (2023)