Key Points
-
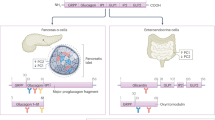
Perturbation of normal homeostatic control of proglucagon processing can occur, which gives rise to production of glucagon-like peptide 1 in the inflamed or injured pancreas
-
The plasticity and interconversion of α cells and β cells provides opportunities for cell replacement and differentiation strategies for the treatment of diabetes mellitus
-
The central role of glucagon in maintenance of euglycaemia underscores the rationale for clinical strategies for simultaneous glucagon and insulin administration for therapy of type 1 diabetes mellitus (T1DM)
-
Glucagon agonists reduce food intake and increase energy expenditure, which highlights pathways that could be targeted in the treatment of obesity
-
Dysregulated glucagon secretion and increased hepatic glucose production in patients with T1DM or type 2 diabetes mellitus forms the basis of attempts to reduce glucagon action to treat these individuals
-
Mechanism-based adverse events, such as cardiovascular effects, are mediated by glucagon receptor signalling and should be considered when developing therapeutic strategies directed at enhancing or attenuating glucagon action
Abstract
Glucagon is secreted from islet α cells and controls blood levels of glucose in the fasting state. Impaired glucagon secretion predisposes some patients with type 1 diabetes mellitus (T1DM) to hypoglycaemia; whereas hyperglycaemia in patients with T1DM or type 2 diabetes mellitus (T2DM) is often associated with hyperglucagonaemia. Hence, therapeutic strategies to safely achieve euglycaemia in patients with diabetes mellitus now encompass bihormonal approaches to simultaneously deliver insulin and glucagon (in patients with T1DM) or reduce excess glucagon action (in patients with T1DM or T2DM). Glucagon also reduces food intake and increases energy expenditure through central and peripheral mechanisms, which suggests that activation of signalling through the glucagon receptor might be useful for controlling body weight. Here, we review new data that is relevant to understanding α-cell biology and glucagon action in the brain, liver, adipose tissue and heart, with attention to normal physiology, as well as conditions associated with dysregulated glucagon action. The feasibility and safety of current and emerging glucagon-based therapies that encompass both gain-of-function and loss-of-function approaches for the treatment of T1DM, T2DM and obesity is discussed in addition to developments, challenges and critical gaps in our knowledge that require additional investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cryer, P. E. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 63, 2188–2195 (2014).
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Pincus, I. J. & Rutman, J. Z. Glucagon, the hyperglycemic agent in pancreatic extracts; a possible factor in certain types of diabetes. Arch. Intern. Med. 92, 666–677 (1953).
Unger, R. H. & Cherrington, A. D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 122, 4–12 (2012).
Cryer, P. E., Davis, S. N. & Shamoon, H. Hypoglycemia in diabetes. Diabetes Care 26, 1902–1912 (2003).
Cryer, P. E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 369, 362–372 (2013).
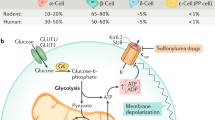
Gromada, J., Franklin, I. & Wollheim, C. B. α-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 28, 84–116 (2007).
Campbell, J. E. & Drucker, D. J. Pharmacology physiology and mechanisms of incretin hormone action. Cell Metab. 17, 819–837 (2013).
Yue, J. T. et al. Amelioration of hypoglycemia via somatostatin receptor type 2 antagonism in recurrently hypoglycemic diabetic rats. Diabetes 62, 2215–2222 (2013).
Zhang, Q. et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab. 18, 871–882 (2013).
Lamy, C. M. et al. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 19, 527–538 (2014).
Plamboeck, A. et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1117–G1127 (2013).
Wewer Albrechtsen, N. J. et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 57, 1919–1926 (2014).
Molina, J. et al. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 63, 2714–2726 (2014).
Rodriguez-Diaz, R. et al. α cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 17, 888–892 (2011).
Unger, R. H. Glucagon physiology and pathophysiology. N. Engl. J. Med. 285, 443–449 (1971).
Gutniak, M., Grill, V. & Efendic, S. Effect of composition of mixed meals—low- versus high-carbohydrate content—on insulin, glucagon, and somatostatin release in healthy humans and in patients with NIDDM. Diabetes Care 9, 244–249 (1986).
Le Marchand, S. J. & Piston, D. W. Glucose suppression of glucagon secretion: metabolic and calcium responses from α-cells in intact mouse pancreatic islets. J. Biol. Chem. 285, 14389–14398 (2010).
Bak, M. J. et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur. J. Endocrinol. 170, 529–538 (2014).
Shah, P. et al. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 85, 4053–4059 (2000).
Kramer, C. K., Borgono, C. A., Van Nostrand, P., Retnakaran, R. & Zinman, B. Glucagon response to oral glucose challenge in type 1 diabetes: lack of impact of euglycemia. Diabetes Care 37, 1076–1082 (2014).
Chia, C. W. et al. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 58, 1342–1349 (2009).
Christensen, M. B., Calanna, S., Holst, J. J., Vilsboll, T. & Knop, F. K. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 99, E418–E426 (2014).
Fujita, Y. et al. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet α-cells and promotes insulin secretion. Gastroenterology 138, 1966–1975 (2010).
Bramswig, N. C. et al. Epigenomic plasticity enables human pancreatic α- to β cell reprogramming. J. Clin. Invest. 123, 1275–1284 (2013).
Nie, Y. et al. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J. Clin. Invest. 105, 955–965 (2000).
Marchetti, P. et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia 55, 3262–3272 (2012).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and α cells. Nat. Med. 17, 1481–1489 (2011).
Nauck, M. A., Vardarli, I., Deacon, C. F., Holst, J. J. & Meier, J. J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54, 10–18 (2010).
Faerch, K. et al. Glucagon-like peptide-1 (GLP-1) response to oral glucose is reduced in pre-diabetes, screen-detected tpe 2 diabetes and obesity, and influenced by sex: The ADDITION-PRO Study. Diabetes http://dx.doi.org/10.2337/db14-1751.
Collombat, P. et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 138, 449–462 (2009).
Sosa-Pineda, B., Chowdhury, K., Torres, M., Oliver, G. & Gruss, P. The Pax4 gene is essential for differentiation of insulin-producing β cells in the mammalian pancreas. Nature 386, 399–402 (1997).
Thorel, F. et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010).
Thorel, F. et al. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes 60, 2872–2882 (2011).
Brereton, M. F. et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat. Commun. 5, 4639 (2014).
Ferrannini, E. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124, 499–508 (2014).
Merovci, A. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124, 509–514 (2014).
Bonner, C. et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic α cells triggers glucagon secretion. Nat. Med. http://dx.doi.org/10.1038/10.1038/nm.3828 (2015).
Jorgensen, N. B. et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 303, E122–E131 (2012).
Meier, J. J. et al. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology 130, 44–54 (2006).
Bahrami, J., Longuet, C., Baggio, L. L., Li, K. & Drucker, D. J. The glucagon-like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob mouse. Gastroenterology 139, 857–868 (2010).
Drucker, D. J. & Yusta, B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu. Rev. Physiol. 76, 561–583 (2014).
Drucker, D. J. & Asa, S. Glucagon gene expression in vertebrate brain. J. Biol. Chem. 263, 13475–13478 (1988).
Campos, R. V., Lee, Y. C. & Drucker, D. J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134, 2156–2164 (1994).
Jin, S.-L. C. et al. Distribution of glucagonlike peptide 1 (GLP-1), glucagon, and glicentin in the rat brain: an immunocytochemical study. J. Comp. Neurol. 271, 519–532 (1988).
Habegger, K. M. et al. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 6, 689–697 (2010).
Tschop, M. H. & DiMarchi, R. D. Outstanding Scientific Achievement Award Lecture 2011: defeating diabesity: the case for personalized combinatorial therapies. Diabetes 61, 1309–1314 (2012).
Mighiu, P. I. et al. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat. Med. 19, 766–772 (2013).
Ali, S. & Drucker, D. J. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 296, E415–E421 (2009).
Tan, T. M. et al. Coadministration of glucagon-like peptide-1 during glucagon infusion in man results in increased energy expenditure and amelioration of hyperglycemia. Diabetes 62, 1131–1138 (2013).
Lee, Y., Wang, M. Y., Du, X. Q., Charron, M. J. & Unger, R. H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60, 391–397 (2011).
Lee, Y. et al. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc. Natl Acad. Sci. USA 109, 14972–14976 (2012).
Gelling, R. W. et al. Lower blood glucose, hyperglucagonemia, and pancreatic α cell hyperplasia in glucagon receptor knockout mice. Proc. Natl Acad. Sci. USA 100, 1438–1443 (2003).
Ali, S., Lamont, B. J., Charron, M. J. & Drucker, D. J. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J. Clin. Invest. 121, 1917–1929 (2011).
Omar, B. A. et al. Fibroblast growth factor 21 (FGF21) and glucagon-like peptide 1 contribute to diabetes resistance in glucagon receptor-deficient mice. Diabetes 63, 101–110 (2014).
Dobbs, R. et al. Glucagon: role in the hyperglycemia of diabetes mellitus. Science 187, 544–547 (1975).
Gerich, J. E. et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N. Engl. J. Med. 292, 985–989 (1975).
Fujikawa, T., Chuang, J. C., Sakata, I., Ramadori, G. & Coppari, R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc. Natl Acad. Sci. USA 107, 17391–17396 (2010).
Perry, R. J. et al. Leptin reverses diabetes by suppression of the hypothalamic–pituitary–adrenal axis. Nat. Med. 20, 759–763 (2014).
Nathan, D. M. et al. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37, 9–16 (2014).
El-Khatib, F. H., Russell, S. J., Nathan, D. M., Sutherlin, R. G. & Damiano, E. R. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci. Transl. Med. 2, 27ra27 (2010).
Russell, S. J. et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N. Engl. J. Med. 371, 313–325 (2014).
Jun, L. S. et al. Absence of glucagon and insulin action reveals a role for the glucagon-like peptide-1 receptor in endogenous glucose production. Diabetes 64, 819–827 (2014).
Gu, W. et al. Glucagon receptor antagonist-mediated improvements in glycemic control are dependent on functional pancreatic GLP-1 receptor. Am. J. Physiol. Endocrinol. Metab. 299, E624–E632 (2010).
Habegger, K. M. et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes 62, 1453–1463 (2013).
Yang, J. et al. Polyomic profiling reveals significant hepatic metabolic alterations in glucagon-receptor (GCGR) knockout mice: implications on anti-glucagon therapies for diabetes. BMC Genomics 12, 281 (2011).
van Dongen, M. G. et al. First proof of pharmacology in humans of a novel glucagon receptor antisense drug. J. Clin. Pharmacol. 55, 298–306 (2015).
Wang, M. Y. et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc. Natl Acad. Sci. USA 112, 2503–2508 (2015).
Sloop, K. W. et al. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J. Clin. Invest. 113, 1571–1581 (2004).
Liang, Y. et al. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 53, 410–417 (2004).
Winzell, M. S. et al. Glucagon receptor antagonism improves islet function in mice with insulin resistance induced by a high-fat diet. Diabetologia 50, 1453–1462 (2007).
Chen, M. et al. Absence of the glucagon-like peptide-1 receptor does not affect the metabolic phenotype of mice with liver-specific Gsα deficiency. Endocrinology 152, 3343–3350 (2011).
Longuet, C. et al. Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes 62, 1196–1205 (2013).
Sinclair, E. M. et al. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology 135, 2096–2106 (2008).
Vuguin, P. M. et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology 147, 3995–4006 (2006).
Zhou, C., Dhall, D., Nissen, N. N., Chen, C. R. & Yu, R. A homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, α cell hyperplasia, and islet cell tumor. Pancreas 38, 941–946 (2009).
Sipos, B. et al. Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J. Clin. Endocrinol. Metab. http://dx.doi.org/10.1210/jc.2014-4405.
Drucker, D. J. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 62, 3316–3323 (2013).
Koehler, J. A. et al. Glucagon-like peptide-1 receptor agonists increase pancreatic mass by induction of protein synthesis. Diabetes 64, 1046–1056 (2015).
Koehler, J. A. et al. GLP-1R agonists promote normal and neoplastic intestinal growth through mechanisms requiring Fgf7. Cell Metab. 21, 379–391 (2015).
Xiao, C., Pavlic, M., Szeto, L., Patterson, B. W. & Lewis, G. F. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60, 383–90 (2011).
Longuet, C. et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 8, 359–371 (2008).
Kawamori, D. et al. Insulin signaling in α cells modulates glucagon secretion in vivo. Cell Metab. 9, 350–361 (2009).
Riddle, M. C. & Drucker, D. J. Emerging therapies mimicking the effects of amylin and glucagon-like peptide 1. Diabetes Care 29, 435–449 (2006).
Silvestre, R. A. et al. Selective amylin inhibition of the glucagon response to arginine is extrinsic to the pancreas. Am. J. Physiol. Endocrinol. Metab. 280, E443–E449 (2001).
Richards, P. et al. Identification and characterisation of glucagon-like peptide-1 receptor expressing cells using a new transgenic mouse model. Diabetes 63, 1224–1233 (2014).
Pyke, C. et al. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155, 1280–1290 (2014).
Dupre, J., Behme, M. T. & McDonald, T. J. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J. Clin. Endocrinol. Metab. 89, 3469–3473 (2004).
Farngren, J., Persson, M., Schweizer, A., Foley, J. E. & Ahren, B. Vildagliptin reduces glucagon during hyperglycemia and sustains glucagon counterregulation during hypoglycemia in type 1 diabetes. J. Clin. Endocrinol. Metab. 97, 3799–3806 (2012).
Aulinger, B. A. et al. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes 63, 1079–1092 (2014).
Cegla, J. et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 63, 3711–3720 (2014).
Billington, C. J., Bartness, T. J., Briggs, J., Levine, A. S. & Morley, J. E. Glucagon stimulation of brown adipose tissue growth and thermogenesis. Am. J. Physiol. 252, R160–R165 (1987).
Kinoshita, K. et al. Glucagon is essential for adaptive thermogenesis in brown adipose tissue. Endocrinology 155, 3484–3492 (2014).
Arafat, A. M. et al. Glucagon increases circulating fibroblast growth factor 21 independently of endogenous insulin levels: a novel mechanism of glucagon-stimulated lipolysis? Diabetologia 56, 588–597 (2013).
Finan, B. et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 21, 27–36 (2015).
Pocai, A. Action and therapeutic potential of oxyntomodulin. Mol. Metab. 14, 241–251 (2013).
Day, J. W. et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757 (2009).
Mukharji, A., Drucker, D. J., Charron, M. J. & Swoap, S. J. Oxyntomodulin increases intrinsic heart rate through the glucagon receptor. Physiol. Rep. 1, e00112 (2013).
Ussher, J. R. & Drucker, D. J. Cardiovascular actions of incretin-based therapies. Circ. Res. 114, 1788–1803 (2014).
Ussher, J. R. et al. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol. Metab. 3, 507–517 (2014).
Ali, S. et al. Cardiomyocyte glucagon receptor signaling modulates outcomes in mice with experimental myocardial infarction. Mol. Metab. 4, 132–143 (2014).
Lockie, S. H. et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 61, 2753–2762 (2012).
Rezania, A. et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes 60, 239–247 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Author information
Authors and Affiliations
Contributions
J.E.C. and D.J.D. researched data for the article, provided substantial contributions to discussions of the content, contributed equally to writing the article, and to reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.J.D. has served as a consultant for companies developing incretin-based therapies for the treatment of diabetes mellitus, including Arisaph Pharmaceuticals, Intarcia Therapeutics, MedImmune, Merck Research Laboratories, Novo Nordisk, NPS Pharmaceuticals and Receptos. Neither D.J.D. nor his family members hold stock directly or indirectly in any of these companies. Preclinical studies in D.J.D.'s laboratory are supported, in part, by grants to Mount Sinai Hospital from Merck, Novo Nordisk and Sanofi. J.E.C. declares no competing interests.
Rights and permissions
About this article
Cite this article
Campbell, J., Drucker, D. Islet α cells and glucagon—critical regulators of energy homeostasis. Nat Rev Endocrinol 11, 329–338 (2015). https://doi.org/10.1038/nrendo.2015.51
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.51
This article is cited by
-
Nonlinear relationship between atherogenic index of plasma and the risk of prediabetes: a retrospective study based on Chinese adults
Cardiovascular Diabetology (2023)
-
Pancreatic alpha cell glucagon–liver FGF21 axis regulates beta cell regeneration in a mouse model of type 2 diabetes
Diabetologia (2023)
-
Pharmacological modulation of adaptive thermogenesis: new clues for obesity management?
Journal of Endocrinological Investigation (2023)
-
Association between osteocalcin, a pivotal marker of bone metabolism, and secretory function of islet beta cells and alpha cells in Chinese patients with type 2 diabetes mellitus: an observational study
Diabetology & Metabolic Syndrome (2022)
-
Dipeptidyl peptidase-4 inhibitor improves glycemic variability in multiple daily insulin-treated type 2 diabetes: a prospective randomized-controlled trial
Diabetology International (2022)