Abstract
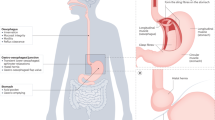
Gastroesophageal reflux disease (GERD) affects 20–30% of the population in Western countries, and is one of the most common clinical problems in daily practice. GERD-associated functional and structural abnormalities are caused by recurrent exposure of the esophagus to acidic and nonacidic refluxate of gastric contents (containing duodenal and intestinal proteases as well as acid and gastric pepsin) from the stomach. Major progress has been made in the understanding of the molecular pathogenesis of GERD-associated mucosal inflammation, suggesting a complex and multifactorial pathogenesis and immune-mediated effects. This Review summarizes the complexity of mucosal pathogenesis, including microscopic changes, mucosal inflammation and GERD-specific molecular mediators, in the context of the clinical features and pathophysiological characteristics of GERD. The abnormal exposure of the esophagus to luminal contents leads to chronic mucosal inflammation that is characterized by the release of IL-8 specifically, as well as other proinflammatory mediators, from the esophageal mucosa. Evidence from animal studies indicates a stepwise inflammatory response by the epithelium, which attracts immune effector cells to infiltrate the mucosa. From bench to bedside, these novel molecular findings might provide new treatment options beyond current acid-suppressive therapy and the principle of inhibition of transient lower esophageal sphincter relaxation.
Key Points
-
GERD-associated mucosal inflammation is characterized by epithelial release of IL-8 and other proinflammatory markers
-
PAR2 expression is upregulated in patients with GERD and induced by acid conditions in cell-culture models; PAR2 activation leads to epithelial IL-8 release and contributes to the pathogenesis of GERD
-
Structural abnormalities and microscopic changes in GERD are characterized by papillary elongation, basal cell hyperplasia, dilated intercellular spaces and an infiltrate of immune cells, and can even be identified using light microscopy
-
Beyond PPI therapy, new pharmacological targets include mechanisms related to transient lower esophageal sphincter relaxation and mechanisms involved in symptom perception (TRPV1, cannabionoid receptors)
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Badreddine, R. J. & Wang, K. K. Barrett esophagus: an update. Nat. Rev. Gastroenterol. Hepatol. 7, 369–378 (2010).
Odze, R. D. Barrett esophagus: histology and pathology for the clinician. Nat. Rev. Gastroenterol. Hepatol. 6, 478–490 (2009).
Dent, J., el-Serag, H. B., Wallander, M. A. & Johansson, S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 54, 710–717 (2005).
el-Serag, H. B. Time trends of gastroesophageal reflux disease: a systematic review. Clin. Gastroenterol. Hepatol. 5, 17–26 (2007).
Wiklund, I. Review of the quality of life and burden of illness in gastroesophageal reflux disease. Dig. Dis. 22, 108–114 (2004).
Kulig, M. et al. Quality of life in relation to symptoms in patients with gastro-oesophageal reflux disease-- an analysis based on the ProGERD initiative. Aliment. Pharmacol. Ther. 18, 767–776 (2003).
Koelz, H. R., Blum, A. L. & Modlin, I. M. Costs of gerd: facts and fiction. Gastroenterology 125, 981–982 (2003).
Dent, J. et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond Study. Gut 59, 714–721 (2010).
Savarino, V., Savarino, E., Parodi, A. & Dulbecco, P. Functional heartburn and non-erosive reflux disease. Dig. Dis. 25, 172–174 (2007).
Locke, G. R. III, Talley, N. J., Fett, S. L., Zinsmeister, A. R. & Melton, L. J. 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 112, 1448–1456 (1997).
Neumann, H., Monkemuller, K., Kandulski, A. & Malfertheiner, P. Dyspepsia and IBS symptoms in patients with NERD, ERD and Barrett's esophagus. Dig. Dis. 26, 243–247 (2008).
Galmiche, J. P. et al. Functional esophageal disorders. Gastroenterology 130, 1459–1465 (2006).
Vela, M. F., Craft, B. M., Sharma, N., Freeman, J. & Hazen-Martin, D. Refractory heartburn: comparison of intercellular space diameter in documented GERD vs. functional heartburn. Am. J. Gastroenterol. 106, 844–850 (2011).
Ronkainen, J. et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand. J. Gastroenterol. 40, 275–285 (2005).
Sharma, P. et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology. 131, 1392–1399 (2006).
Jankowski, J. A., Provenzale, D. & Moayyedi, P. Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the west. Gastroenterology 122, 588–590 (2002).
Yousef, F. et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am. J. Epidemiol. 168, 237–249 (2008).
Labenz, J. et al. Prospective follow-up data from the ProGERD study suggest that GERD is not a categorial disease. Am. J. Gastroenterol. 101, 2457–2462 (2006).
Malfertheiner, P. et al. Evolution of gastroesophageal reflux disease (GERD) over 5 years under routine medical care—the ProGERD study. Aliment. Pharmacol. Ther. (in press).
Orenstein, S. R., Shalaby, T. M., Kelsey, S. F. & Frankel, E. Natural history of infant reflux esophagitis: symptoms and morphometric histology during one year without pharmacotherapy. Am. J. Gastroenterol. 101, 628–640 (2006).
Sherman, P. M. et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am. J. Gastroenterol. 104, 1278–1295 (2009).
Vakil, N., van Zanten, S. V., Kahrilas, P., Dent, J. & Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am. J. Gastroenterol. 101, 1900–1920 (2006).
Sifrim, D. & Holloway, R. Transient lower esophageal sphincter relaxations: how many or how harmful? Am. J. Gastroenterol. 96, 2529–2532 (2001).
Jacobson, B. C., Somers, S. C., Fuchs, C. S., Kelly, C. P. & Camargo, C. A. Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N. Engl. J. Med. 354, 2340–2348 (2006).
Hampel, H., Abraham, N. S. & el-Serag, H. B. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 143, 199–211 (2005).
Barlow, W. J. & Orlando, R. C. The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology 128, 771–778 (2005).
Modlin, I. M. et al. Diagnosis and management of non-erosive reflux disease - the Vevey NERD Consensus Group. Digestion 80, 74–88 (2009).
Kahrilas, P. J. & Smout, A. J. Esophageal disorders. Am. J. Gastroenterol. 105, 747–756 (2010).
Dean, B. B., Gano, A. D. Jr, Knight, K., Ofman, J. J. & Fass, R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin. Gastroenterol. Hepatol. 2, 656–664 (2004).
Fass, R. & Sifrim, D. Management of heartburn not responding to proton pump inhibitors. Gut 58, 295–309 (2009).
Smout, A. J. The patient with GORD and chronically recurrent problems. Best Pract. Res. Clin. Gastroenterol. 21, 365–378 (2007).
Smith, J. L., Opekun, A. R., Larkai, E. & Graham, D. Y. Sensitivity of the esophageal mucosa to pH in gastroesophageal reflux disease. Gastroenterology 96, 683–689 (1989).
Martinez, S. D., Malagon, I. B., Garewal, H. S., Cui, H. & Fass, R. Non-erosive reflux disease (NERD)--acid reflux and symptom patterns. Aliment. Pharmacol. Ther. 17, 537–545 (2003).
Sifrim, D., Castell, D., Dent, J. & Kahrilas, P. J. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 53, 1024–1031 (2004).
Agrawal, A. et al. Symptoms with acid and nonacid reflux may be produced by different mechanisms. Dis. Esophagus 22, 467–470 (2009).
Vela, M. F. et al. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology 120, 1599–1606 (2001).
Sifrim, D. et al. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24-hour pH-impedance recordings. Gastroenterology 120, 1588–1598 (2001).
Bredenoord, A. J., Weusten, B. L., Curvers, W. L., Timmer, R. & Smout, A. J. Determinants of perception of heartburn and regurgitation. Gut 55, 313–318 (2006).
Mainie, I. et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 55, 1398–1402 (2006).
Sharma, N., Agrawal, A., Freeman, J., Vela, M. F. & Castell, D. An analysis of persistent symptoms in acid-suppressed patients undergoing impedance-pH monitoring. Clin. Gastroenterol. Hepatol. 6, 521–524 (2008).
Hemmink, G. J. et al. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: 'on' or 'off' proton pump inhibitor? Am. J. Gastroenterol. 103, 2446–2453 (2008).
Furuta, T. et al. Effect of cytochrome P4502C19 genotypic differences on cure rates for gastroesophageal reflux disease by lansoprazole. Clin. Pharmacol. Ther. 72, 453–460 (2002).
Furuta, T. et al. CYP2C19 genotype is associated with symptomatic recurrence of GERD during maintenance therapy with low-dose lansoprazole. Eur. J. Clin. Pharmacol. 65, 693–698 (2009).
Fass, R., Shapiro, M., Dekel, R. & Sewell, J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease—where next? Aliment. Pharmacol. Ther. 22, 79–94 (2005).
Ismail-Beigi, F., Horton, P. F. & Pope, C. E. Histological consequences of gastroesophageal reflux in man. Gastroenterology 58, 163–174 (1970).
Hopwood, D., Milne, G. & Logan, K. R. Electron microscopic changes in human oesophageal epithelium in oesophagitis. J. Pathol. 129, 161–167 (1979).
Tobey, N. A., Carson, J. L., Alkiek, R. A. & Orlando, R. C. Dilated intercellular spaces: a morphological feature of acid reflux—damaged human esophageal epithelium. Gastroenterology 111, 1200–1205 (1996).
Tobey, N. A. et al. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am. J. Gastroenterol. 99, 13–22 (2004).
Vieth, M. et al. Radial distribution of dilated intercellular spaces of the esophageal squamous epithelium in patients with reflux disease exhibiting discrete endoscopic lesions. Dig. Dis. 22, 208–212 (2004).
Vieth, M. et al. What parameters are relevant for the histological diagnosis of gastroesophageal reflux disease without Barrett's mucosa? Dig. Dis. 22, 196–201 (2004).
Zentilin, P. et al. Carditis in patients with gastro-oesophageal reflux disease: results of a controlled study based on both endoscopy and 24-h oesophageal pH monitoring. Aliment. Pharmacol. Ther. 19, 1285–1292 (2004).
Fiocca, R. et al. Development of consensus guidelines for the histologic recognition of microscopic esophagitis in patients with gastroesophageal reflux disease: the Esohisto project. Hum. Pathol. 41, 223–231 (2010).
Yerian, L. et al. Refinement and reproducibility of histologic criteria for the assessment of microscopic lesions in patients with gastroesophageal reflux disease: the esohisto project. Dig. Dis. Sci. 56, 2656–2665 (2011).
Fiocca, R. et al. Long-term outcome of microscopic esophagitis in chronic GERD patients treated with esomeprazole or laparoscopic antireflux surgery in the LOTUS trial. Am. J. Gastroenterol. 105, 1015–1023 (2010).
Galmiche, J. P. et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 305, 1969–1977 (2011).
Farre, R. et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut 57, 1366–1374 (2008).
Tobey, N. A., Gambling, T. M., Vanegas, X. C., Carson, J. L. & Orlando, R. C. Physicochemical basis for dilated intercellular spaces in non-erosive acid-damaged rabbit esophageal epithelium. Dis. Esophagus 21, 757–764 (2008).
Carney, C. N., Orlando, R. C., Powell, D. W. & Dotson, M. M. Morphologic alterations in early acid-induced epithelial injury of the rabbit esophagus. Lab. Invest. 45, 198–208 (1981).
Farre, R. et al. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut 59, 164–169 (2010).
Farre, R. et al. Evaluation of oesophageal mucosa integrity by the intraluminal impedance technique. Gut 60, 885–892 (2011).
Haggitt, R. C. Histopathology of reflux-induced esophageal and supraesophageal injuries. Am. J. Med. 108 (Suppl. 4a), 109S–111S (2000).
Odze, R. D. Unraveling the mystery of the gastroesophageal junction: a pathologist's perspective. Am. J. Gastroenterol. 100, 1853–1867 (2005).
Tummala, V., Barwick, K. W., Sontag, S. J., Vlahcevic, R. Z. & McCallum, R. W. The significance of intraepithelial eosinophils in the histologic diagnosis of gastroesophageal reflux. Am. J. Clin. Pathol. 87, 43–48 (1987).
Livstone, E. M., Sheahan, D. G. & Behar, J. Studies of esophageal epithelial cell proliferation in patients with reflux esophagitis. Gastroenterology 73, 1315–1319 (1977).
Tobey, N. A. et al. The role of pepsin in acid injury to esophageal epithelium. Am. J. Gastroenterol. 96, 3062–3070 (2001).
Orlando, R. C. Pathophysiology of gastroesophageal reflux disease. J. Clin. Gastroenterol. 42, 584–588 (2008).
Fitzgerald, R. C. Inflammation at the neo squamocolumnar junction in Barrett's oesophagus. Gut 47, 870 (2000).
Fitzgerald, R. C. et al. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50, 451–459 (2002).
Kandulski, A. et al. Chronic mucosal inflammation of the gastric cardia in gastroesophageal reflux disease is not regulated by FOXP3-expressing T cells. Dig. Dis. Sci. 54, 1940–1946 (2009).
Isomoto, H. et al. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am. J. Gastroenterol. 98, 551–556 (2003).
Yoshida, N. et al. Interleukin-8 production via protease-activated receptor 2 in human esophageal epithelial cells. Int. J. Mol. Med. 19, 335–340 (2007).
Monkemuller, K. et al. Interleukin-1β and interleukin-8 expression correlate with the histomorphological changes in esophageal mucosa of patients with erosive and non-erosive reflux disease. Digestion 79, 186–195 (2009).
Yamaguchi, T. et al. Cytokine-induced neutrophil accumulation in the pathogenesis of acute reflux esophagitis in rats. Int. J. Mol. Med. 16, 71–77 (2005).
Rieder, F. et al. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology 132, 154–165 (2007).
Cheng, L. et al. HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1307–G1317 (2006).
Isomoto, H. et al. Enhanced expression of interleukin-8 and activation of nuclear factor kappa-B in endoscopy-negative gastroesophageal reflux disease. Am. J. Gastroenterol. 99, 589–597 (2004).
Oh, D. S. et al. Reduction of interleukin 8 gene expression in reflux esophagitis and Barrett's esophagus with antireflux surgery. Arch. Surg. 142, 554–559 (2007).
Mukaida, N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L566–L577 (2003).
Hamaguchi, M. et al. Increased expression of cytokines and adhesion molecules in rat chronic esophagitis. Digestion 68, 189–197 (2003).
Cheng, L. et al. Acid-induced release of platelet-activating factor by human esophageal mucosa induces inflammatory mediators in circular smooth muscle. J. Pharmacol. Exp. Ther. 319, 117–126 (2006).
Cheng, L. et al. In vitro model of acute esophagitis in the cat. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G860–G869 (2005).
Rieder, F., Biancani, P., Harnett, K., Yerian, L. & Falk, G. W. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G571–G581 (2010).
Kandulski, A. et al. Proteinase-activated receptor-2 in the pathogenesis of gastroesophageal reflux disease. Am. J. Gastroenterol. 105, 1934–1943 (2010).
Souza, R. F. et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137, 1776–1784 (2009).
Yoshida, N. et al. Interleukin-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand. J. Gastroenterol. 39, 816–822 (2004).
Steinhoff, M. et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 6, 151–158 (2000).
Soreide, K. Proteinase-activated receptor 2 (PAR-2) in gastrointestinal and pancreatic pathophysiology, inflammation and neoplasia. Scand. J. Gastroenterol. 43, 902–909 (2008).
Scarborough, R. M. et al. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J. Biol. Chem. 267, 13146–13149 (1992).
Dery, O., Corvera, C. U., Steinhoff, M. & Bunnett, N. W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 274, C1429–C1452 (1998).
Cenac, N. et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am. J. Pathol. 161, 1903–1915 (2002).
Vergnolle, N. et al. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat. Med. 7, 821–826 (2001).
Coelho, A. M., Vergnolle, N., Guiard, B., Fioramonti, J. & Bueno, L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology 122, 1035–1047 (2002).
Jacob, C. et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and β-arrestins. J. Biol. Chem. 280, 31936–31948 (2005).
Soderholm, J. D. Stress-related changes in oesophageal permeability: filling the gaps of GORD? Gut 56, 1177–1180 (2007).
Souza, R. F. Bringing GERD management up to PAR-2. Am. J. Gastroenterol. 105, 1944–1946 (2010).
Naito, Y. et al. Role of pancreatic trypsin in chronic esophagitis induced by gastroduodenal reflux in rats. J. Gastroenterol. 41, 198–208 (2006).
Amadesi, S. et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 24, 4300–4312 (2004).
Bhat, Y. M. & Bielefeldt, K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur. J. Gastroenterol. Hepatol. 18, 263–270 (2006).
Matthews, P. J. et al. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur. J. Gastroenterol. Hepatol. 16, 897–902 (2004).
Guarino, M. P. et al. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol. Motil. 22, 746–751, e219 (2010).
Cortright, D. N. & Szallasi, A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur. J. Biochem. 271, 1814–1819 (2004).
Kindt, S., Vos, R., Blondeau, K. & Tack, J. Influence of intra-oesophageal capsaicin instillation on heartburn induction and oesophageal sensitivity in man. Neurogastroenterol. Motil. 21, 1032–e82 (2009).
Yiangou, Y. et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 357, 1338–1339 (2001).
Cheng, L. et al. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G135–G143 (2009).
Ma, J., Harnett, K. M., Behar, J., Biancani, P. & Cao, W. Signaling in TRPV1-induced platelet activating factor (PAF) in human esophageal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G233–G240 (2010).
Shieh, K. R. et al. Evidence for neurotrophic factors associating with TRPV1 gene expression in the inflamed human esophagus. Neurogastroenterol. Motil. 22, 971–7, e252 (2010).
Koek, G. H., Sifrim, D., Lerut, T., Janssens, J. & Tack, J. Effect of the GABAB agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 52, 1397–1402 (2003).
Vela, M. F., Tutuian, R., Katz, P. O. & Castell, D. O. Baclofen decreases acid and non-acid post-prandial gastro-oesophageal reflux measured by combined multichannel intraluminal impedance and pH. Aliment. Pharmacol. Ther. 17, 243–251 (2003).
Gerson, L. B. et al. Arbaclofen placarbil decreases postprandial reflux in patients with gastroesophageal reflux disease. Am. J. Gastroenterol. 105, 1266–1275 (2010).
Boeckxstaens, G. E. et al. Effects of lesogaberan on reflux and lower esophageal sphincter function in patients with gastroesophageal reflux disease. Gastroenterology 139, 409–417 (2010).
Zerbib, F. et al. Randomised clinical trial: effects of monotherapy with ADX10059, a mGluR5 inhibitor, on symptoms and reflux events in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 33, 911–921 (2011).
Zerbib, F., Keywood, C. & Strabach, G. Efficacy, tolerability and pharmacokinetics of a modified release formulation of ADX10059, a negative allosteric modulator of metabotropic glutamate receptor 5: an esophageal pH-impedance study in healthy subjects. Neurogastroenterol. Motil. 22, 859–865, e231 (2010).
Keywood, C., Wakefield, M. & Tack, J. A proof-of-concept study evaluating the effect of ADX10059, a metabotropic glutamate receptor-5 negative allosteric modulator, on acid exposure and symptoms in gastro-oesophageal reflux disease. Gut 58, 1192–1199 (2009).
Ruth, M., Hamelin, B., Rohss, K. & Lundell, L. The effect of mosapride, a novel prokinetic, on acid reflux variables in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 12, 35–40 (1998).
Ruth, M., Finizia, C., Cange, L. & Lundell, L. The effect of mosapride on oesophageal motor function and acid reflux in patients with gastro-oesophageal reflux disease. Eur. J. Gastroenterol. Hepatol. 15, 1115–1121 (2003).
Cho, Y. K. et al. The effect of mosapride on esophageal motility and bolus transit in asymptomatic volunteers. J. Clin. Gastroenterol. 40, 286–292 (2006).
Lehmann, A. et al. Cannabinoid receptor agonism inhibits transient lower esophageal sphincter relaxations and reflux in dogs. Gastroenterology 123, 1129–1134 (2002).
Partosoedarso, E. R., Abrahams, T. P., Scullion, R. T., Moerschbaecher, J. M. & Hornby, P. J. Cannabinoid1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J. Physiol. 550, 149–158 (2003).
Beaumont, H. et al. Effect of delta9-tetrahydrocannabinol, a cannabinoid receptor agonist, on the triggering of transient lower oesophageal sphincter relaxations in dogs and humans. Br. J. Pharmacol. 156, 153–162 (2009).
Scarpellini, E. et al. Effect of rimonabant on oesophageal motor function in man. Aliment. Pharmacol. Ther. 33, 730–737 (2011).
Broekaert, D., Fischler, B., Sifrim, D., Janssens, J. & Tack, J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: a double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 23, 365–370 (2006).
Krarup, A. L. et al. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment. Pharmacol. Ther. 33, 1113–1122 (2011).
Author information
Authors and Affiliations
Contributions
A. Kandulski researched data for the article. Both authors contributed equally to discussions of content, writing, reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
P. Malfertheiner declares that he has received grant or research support from Movetis and Novartis. A. Kandulski declares no competing interests.
Rights and permissions
About this article
Cite this article
Kandulski, A., Malfertheiner, P. Gastroesophageal reflux disease—from reflux episodes to mucosal inflammation. Nat Rev Gastroenterol Hepatol 9, 15–22 (2012). https://doi.org/10.1038/nrgastro.2011.210
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.210
This article is cited by
-
Tojapride Reverses Esophageal Epithelial Inflammatory Responses on Reflux Esophagitis Model Rats
Chinese Journal of Integrative Medicine (2021)
-
Impact of Gastroesophageal Reflux Disease on Mucosal Immunity and Atopic Disorders
Clinical Reviews in Allergy & Immunology (2019)
-
Candidate serum metabolite biomarkers for differentiating gastroesophageal reflux disease, Barrett’s esophagus, and high-grade dysplasia/esophageal adenocarcinoma
Metabolomics (2017)
-
Association of interleukin-1B gene polymorphisms at site 511 with gastroesophageal reflux disease: a meta-analysis
Esophagus (2016)
-
Wie ist es um die Behandlung von Patienten mit gastroösophagealen Refluxsymptomen in Deutschland bestellt?
MMW - Fortschritte der Medizin (2015)