Abstract
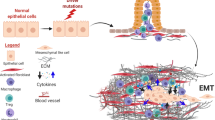
Pancreatic ductal adenocarcinoma (PDAC) is one of the five most lethal malignancies worldwide and survival has not improved substantially in the past 30 years. Desmoplasia (abundant fibrotic stroma) is a typical feature of PDAC in humans, and stromal activation commonly starts around precancerous lesions. It is becoming clear that this stromal tissue is not a bystander in disease progression. Cancer–stroma interactions effect tumorigenesis, angiogenesis, therapy resistance and possibly the metastatic spread of tumour cells. Therefore, targeting the tumour stroma, in combination with chemotherapy, is a promising new option for the treatment of PDAC. In this Review, we focus on four issues. First, how can stromal activity be used to detect early steps of pancreatic carcinogenesis? Second, what is the effect of perpetual pancreatic stellate cell activity on angiogenesis and tissue perfusion? Third, what are the (experimental) antifibrotic therapy options in PDAC? Fourth, what lessons can be learned from Langton's Ant (a simple mathematical model) regarding the unpredictability of genetically engineered mouse models?
Key Points
-
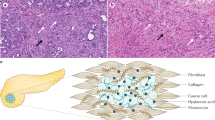
Desmoplastic stroma is a typical feature of pancreatic ductal adenocarcinoma (PDAC); it is either absent or much less prominent in other tumours of the pancreas
-
Precursor lesions of PDAC (such as pancreatic intraepithelial neoplasms and atypical flat lesions) elicit stromal activation and extracellular matrix deposition around them
-
Precursor lesions of PDAC are beneath the detection limit of conventional diagnostic methods; late diagnosis of PDAC leads to a closed therapeutic time window, thus hindering curative treatment
-
The activated stroma of pancreatic cancer has an effect on the aggressiveness of the tumour as well as its resistance to therapy
-
Activated stroma is a new diagnostic and therapeutic target in the treatment of PDAC
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Raimondi, S. et al. Epidemiology of pancreatic cancer: an overview. Nat. Rev. Gastroenterol. Hepatol. 6, 699–708 (2009).
Winter, J. M. et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann. Surg. Oncol. 19, 169–175 (2012).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Conroy, T. et al. Metastatic pancreatic cancer: old drugs, new paradigms. Curr. Opin. Oncol. 23, 390–395 (2011).
Kessenbrock, K. et al. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Kindler, H. L. et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 28, 3617–3622 (2010).
Overall, C. M. et al. Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 6, 227–239 (2006).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Moore, M. J. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 25, 1960–1966 (2007).
Erkan, M. et al. The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr. Mol. Med. 12, 288–303 (2012).
Erkan, M. et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 6, 1155–1161 (2008).
Mollenhauer, J., Roether, I. & Kern, H. F. Distribution of extracellular matrix proteints in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas 2, 14–24 (1987).
Apte, M. V. et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43, 128–133 (1998).
Apte, M. V. et al. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J. Gastroenterol. Hepatol. 27 (Suppl. 2), 69–74 (2012).
Bachem, M. G. et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115, 421–432 (1998).
Bachem, M. G. et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128, 907–921 (2005).
Erkan, M. et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61, 172–178 (2012).
Erkan, M. et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 132, 1447–1464 (2007).
Erkan, M. et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia 11, 497–508 (2009).
Hwang, R. F. et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 (2008).
Paron, I. et al. Tenascin-C enhances pancreatic cancer cell growth and motility and affects cell adhesion through activation of the integrin pathway. PLoS ONE 6, e21684 (2011).
Vonlaufen, A. et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 68, 2085–2093 (2008).
Omary, M. B., Lugea, A., Lowe, A. W. & Pandol, S. J. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J. Clin. Invest. 117, 50–59 (2007).
Apte, M. V. & Wilson, J. S. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J. Gastroenterol. Hepatol. 27 (Suppl. 2), 69–74 (2012).
Kelsen, D. P. et al. Pain and depression in patients with newly diagnosed pancreas cancer. J. Clin. Oncol. 13, 748–755 (1995).
Klein, A. P. et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 64, 2634–2638 (2004).
Giardiello, F. M. et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 119, 1447–1453 (2000).
Lowenfels, A. B. et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med. 328, 1433–1437 (1993).
Canto, M. I. et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142, 796–804 (2012).
Holzapfel, K. et al. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdom. Imaging 36, 179–184 (2011).
Aichler, M. et al. Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J. Pathol. 226, 723–734 (2011).
Brune, K. et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 30, 1067–1076 (2006).
Pérez-Mancera, P. A. et al. What we have learned about pancreatic cancer from mouse models. Gastroenterology 142, 1079–1092 (2012).
Hernandez-Munoz, I. et al. Pancreatic ductal adenocarcinoma: cellular origin, signaling pathways and stroma contribution. Pancreatology 8, 462–469 (2008).
Pour, P. Islet cells as a component of pancreatic ductal neoplasms. I. Experimental study: ductular cells, including islet cell precursors, as primary progenitor cells of tumors. Am. J. Pathol. 90, 295–316 (1978).
Kong, B. et al. AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-β-mediated ERK signaling. Oncogene 29, 5146–5158 (2010).
Hruban, R. H. et al. Update on pancreatic intraepithelial neoplasia. Int. J. Clin. Exp. Pathol. 1, 306–316 (2008).
Matthaei, H. et al. Cystic precursors to invasive pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 8, 141–150 (2011).
Salvia, R. et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann. Surg. 239, 678–685 (2004).
Sohn, T. A. et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann. Surg. 239, 788–797 (2004).
Yamao, K. et al. The prognosis of intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology 47, 1129–1134 (2000).
Maire, F. et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut 51, 717–722 (2002).
Stanger, B. Z. et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 8, 185–195 (2005).
Wagner, M. et al. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 15, 286–293 (2001).
Erkan, M. et al. Tumor microenvironment and progression of pancreatic cancer. Exp. Oncol. 32, 128–131 (2010).
Eser, S. et al. In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging. Proc. Natl Acad. Sci. USA 108, 9945–9950 (2011).
Guerra, C. et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302 (2007).
Barcellos-Hoff, M. H. et al. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60, 1254–1260 (2000).
Radisky, E. S. et al. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland Biol. Neoplasia 15, 201–212 (2010).
Sternlicht, M. D. et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98, 137–146 (1999).
Guturu, P. et al. Interplay of tumor microenvironment cell types with parenchymal cells in pancreatic cancer development and therapeutic implications. J. Gastrointest. Cancer 40, 1–9 (2009).
Masamune, A. et al. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut 58, 550–559 (2009).
Pandol, S. et al. Desmoplasia of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 7 (11 Suppl.), S44–S47 (2009).
Radisky, D. C. et al. Matrix metalloproteinase-induced fibrosis and malignancy in breast and lung. Proc. Am. Thorac. Soc. 5, 316–322 (2008).
Erkan, M. et al. Organ-, inflammation- and cancer specific transcriptional fingerprints of pancreatic and hepatic stellate cells. Mol. Cancer 9, 88 (2010).
Pilarsky, C. et al. Activation of Wnt signalling in stroma from pancreatic cancer identified by gene expression profiling. J. Cell. Mol. Med. 12, 2823–2835 (2008).
Masamune, A. et al. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G709–G717 (2008).
Jacobetz, M. A. et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut http://dx.doi.org/10.1136/gutjnl-2012-302529.
Samkharadze, T. et al. Pigment epithelium-derived factor associates with neuropathy and fibrosis in pancreatic cancer. Am. J. Gastroenterol. 106, 968–980 (2011).
Herrmann, K. et al. Comparison of 3'-deoxy-3'-[(18)F]fluorothymidine positron emission tomography (FLT PET) and FDG PET/CT for the detection and characterization of pancreatic tumours. Eur. J. Nucl. Med. Mol. Imaging 39, 846–851 (2012).
Bausch, D. et al. Plectin-1 as a novel biomarker for pancreatic cancer. Clin. Cancer Res. 17, 302–309 (2011).
Cruz-Monserrate, Z. et al. Detection of pancreatic cancer tumours and precursor lesions by cathepsin E activity in mouse models. Gut http://dx.doi.org/10.1136/gutjnl-2011-300544.
de Visser, M. et al. Novel 111In-labelled bombesin analogues for molecular imaging of prostate tumours. Eur. J. Nucl. Med. Mol. Imaging 34, 1228–1238 (2007).
Delrue, L. J. et al. Assessment of neovascular permeability in a pancreatic tumor model using dynamic contrast-enhanced (DCE) MRI with contrast agents of different molecular weights. MAGMA 24, 225–232 (2011).
Djidja, M. C. et al. MALDI-ion mobility separation-mass spectrometry imaging of glucose-regulated protein 78 kDa (Grp78) in human formalin-fixed, paraffin-embedded pancreatic adenocarcinoma tissue sections. J. Proteome Res. 8, 4876–4884 (2009).
Hilderbrand, S. A. et al. Near infrared fluorescence-based bacteriophage particles for ratiometric pH imaging. Bioconjug. Chem. 19, 1635–1639 (2008).
Kelly, K. A. et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 5, e85 (2008).
Kim, Y. H. et al. An RNA aptamer that specifically binds pancreatic adenocarcinoma up-regulated factor inhibits migration and growth of pancreatic cancer cells. Cancer Lett. 313, 76–83 (2011).
Kimura, R. H. et al. Pharmacokinetically stabilized cystine knot peptides that bind alpha-v-beta-6 integrin with single-digit nanomolar affinities for detection of pancreatic cancer. Clin. Cancer Res. 18, 839–849 (2012).
Morse, D. L. et al. Identification of novel pancreatic adenocarcinoma cell-surface targets by gene expression profiling and tissue microarray. Biochem. Pharmacol. 80, 748–754 (2010).
Partecke, I. L. et al. In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. BMC Cancer 11, 40 (2011).
Rialon, K. L. et al. Aptamers: potential applications to pancreatic cancer therapy. Anticancer Agents Med. Chem. 11, 434–441 (2011).
Yang, L. et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology 136, 1514–1525.e2 (2009).
Zaman, M. B. et al. Single-domain antibody bioconjugated near-IR quantum dots for targeted cellular imaging of pancreatic cancer. J. Nanosci. Nanotechnol. 11, 3757–3763 (2011).
Farace, P. et al. DCE-MRI using small-molecular and albumin-binding contrast agents in experimental carcinomas with different stromal content. Eur. J. Radiol. 78, 52–59 (2011).
Fukushima, N. et al. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod. Pathol. 21, 1044–1053 (2008).
Phillips, P. A. et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc. Natl Acad. Sci. USA 107, 17397–17402 (2010).
Masamune, A. et al. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G99–G108 (2008).
Zhang, W. et al. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol. Ther. 6, 218–227 (2007).
Jiang, X. et al. Pancreatic islet and stellate cells are the main sources of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in pancreatic cancer. Pancreatology 9, 165–172 (2009).
Masamune, A. et al. Pancreatic stellate cells express Toll-like receptors. J. Gastroenterol. 43, 352–362 (2008).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Xu, Z. et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am. J. Pathol. 177, 2585–2596 (2010).
Esposito, I. et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J. Clin. Pathol. 57, 630–636 (2004).
Folkman, J. Angiogenesis. Annu. Rev. Med. 57, 1–18 (2006).
Reiser-Erkan, C. et al. Hypoxia-inducible proto-oncogene Pim-1 is a prognostic marker in pancreatic ductal adenocarcinoma. Cancer Biol. Ther. 7, 1352–1359 (2008).
Michalski, C. W. et al. Mononuclear cells modulate the activity of pancreatic stellate cells which in turn promote fibrosis and inflammation in chronic pancreatitis. J. Transl. Med. 5, 63 (2007).
Michalski, C. W. et al. Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells. PLoS ONE 3, e1701 (2008).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Von Hoff, D. D. et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J. Clin. Oncol. 29, 4548–4554 (2011).
Oparil, S. et al. The renin-angiotensin system (second of two parts). N. Engl. J. Med. 291, 446–457 (1974).
Oparil, S. et al. The renin-angiotensin system (first of two parts). N. Engl. J. Med. 291, 389–401 (1974).
Peach, M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol. Rev. 57, 313–370 (1977).
Paul, M. et al. Physiology of local renin-angiotensin systems. Physiol. Rev. 86, 747–803 (2006).
Tsang, S. W. et al. The role of the pancreatic renin-angiotensin system in acinar digestive enzyme secretion and in acute pancreatitis. Regul. Pept. 119, 213–219 (2004).
Tsang, S. W. et al. Prophylactic and therapeutic treatments with AT 1 and AT 2 receptor antagonists and their effects on changes in the severity of pancreatitis. Int. J. Biochem. Cell Biol. 36, 330–339 (2004).
Kuno, A. et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology 124, 1010–1019 (2003).
Ulmasov, B. et al. Protective role of angiotensin II type 2 receptor signaling in a mouse model of pancreatic fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G284–G294 (2009).
Sakurai, T. et al. Involvement of angiotensin II and reactive oxygen species in pancreatic fibrosis. Pancreatology 11 (Suppl. 2), 7–13 (2011).
Ulmasov, B. et al. Angiotensin II signaling through the AT1a and AT1b receptors does not have a role in the development of cerulein-induced chronic pancreatitis in the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G70–G80 (2010).
Ernsberger, P. et al. Metabolic actions of angiotensin receptor antagonists: PPAR-gamma agonist actions or a class effect? Curr. Opin. Pharmacol. 7, 140–145 (2007).
Ling, M. T. et al. Tocotrienol as a potential anticancer agent. Carcinogenesis 33, 233–239 (2012).
Li, X. C. et al. alpha-Tocopherol treatment ameliorates chronic pancreatitis in an experimental rat model induced by trinitrobenzene sulfonic acid. Pancreatology 11, 5–11 (2011).
Rickmann, M. et al. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology 132, 2518–2532 (2007).
Jiang, F. et al. Comparison of antioxidative and antifibrotic effects of alpha-tocopherol with those of tocotrienol-rich fraction in a rat model of chronic pancreatitis. Pancreas 40, 1091–1096 (2011).
Priyadarsini, K. I. et al. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 50, 2200–2206 (2002).
Iino, T. et al. Effect of ellagic acid on gastric damage induced in ischemic rat stomachs following ammonia or reperfusion. Life Sci. 70, 1139–1150 (2002).
Thresiamma, K. C. et al. Inhibition of liver fibrosis by ellagic acid. Indian J. Physiol. Pharmacol. 40, 363–366 (1996).
Masamune, A. et al. Ellagic acid blocks activation of pancreatic stellate cells. Biochem. Pharmacol. 70, 869–878 (2005).
Suzuki, N. et al. Ellagic acid inhibits pancreatic fibrosis in male Wistar Bonn/Kobori rats. Dig. Dis. Sci. 54, 802–810 (2009).
Ratnam, D. V. et al. Simultaneous analysis of ellagic acid and coenzyme Q(10) by derivative spectroscopy and HPLC. Talanta 70, 387–391 (2006).
Kitamura, M. et al. Epigallocatechin gallate suppresses peritoneal fibrosis in mice. Chem. Biol. Interact. 195, 95–104 (2012).
Kim, N. et al. Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut 58, 1382–1390 (2009).
Masamune, A. et al. Signal transduction in pancreatic stellate cells. J. Gastroenterol. 44, 249–260 (2009).
Kunz-Schughart, L. A. et al. A heterologous 3-D coculture model of breast tumor cells and fibroblasts to study tumor-associated fibroblast differentiation. Exp. Cell Res. 266, 74–86 (2001).
Mersmann, M. et al. Human antibody derivatives against the fibroblast activation protein for tumor stroma targeting of carcinomas. Int. J. Cancer 92, 240–248 (2001).
Cheng, J. D. et al. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 62, 4767–4772 (2002).
Loeffler, M. et al. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Invest. 116, 1955–1962 (2006).
Kraman, M. et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330, 827–830 (2010).
Morris, J. P. 4th, Wang, S. C. & Hebrok, M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat. Rev. Cancer 10, 683–695 (2010).
Yauch, R. L. et al. A paracrine requirement for hedgehog signalling in cancer. Nature 455, 406–410 (2008).
Tian, H. et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc. Natl Acad. Sci. USA 106, 4254–4259 (2009).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Pinzani, M. Pancreatic stellate cells: new kids become mature. Gut 55, 12–14 (2006).
Langton, C. Studying artificial life with cellular automata. Physica D: Nonlinear Phenomena 22, 120–149 (1986).
Branda, C. S. et al. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004).
Acknowledgements
This work was supported in part by a grant from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung [BMBF] to M. Erkan, C. W. Michalski and J. Kleeff) within the “National Genome Research Network” (NGFN-Plus; 01GS08115).
Author information
Authors and Affiliations
Contributions
M. Erkan and C. W. Michalski contributed to all aspects of the manuscript. S. Hausmann researched data for the article and contributed to writing and reviewing/editing the manuscript. A. A. Fingerle and M. Dobritz contributed to discussion of content, writing and reviewing/editing of the manuscript. J. Kleeff and H. Friess contributed to discussion of content and reviewing/editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Erkan, M., Hausmann, S., Michalski, C. et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol 9, 454–467 (2012). https://doi.org/10.1038/nrgastro.2012.115
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.115
This article is cited by
-
Immune checkpoint inhibitors in gastrointestinal malignancies: an Umbrella review
Cancer Cell International (2024)
-
Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: new opportunities in cancer immunotherapy and advances in clinical trials
Molecular Cancer (2023)
-
An ultra-small bispecific protein augments tumor penetration and treatment for pancreatic cancer
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Comparing the clinical value of baseline [68 Ga]Ga-FAPI-04 PET/CT and [18F]F-FDG PET/CT in pancreatic ductal adenocarcinoma: additional prognostic value of the distal pancreatitis
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Comparison of the diagnostic efficacy of 68 Ga-FAPI-04 PET/MR and 18F-FDG PET/CT in patients with pancreatic cancer
European Journal of Nuclear Medicine and Molecular Imaging (2022)