Abstract
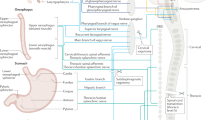
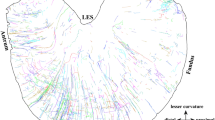
Visceral sensory neurons activate reflex pathways that control gut function and also give rise to important sensations, such as fullness, bloating, nausea, discomfort, urgency and pain. Sensory neurons are organised into three distinct anatomical pathways to the central nervous system (vagal, thoracolumbar and lumbosacral). Although remarkable progress has been made in characterizing the roles of many ion channels, receptors and second messengers in visceral sensory neurons, the basic aim of understanding how many classes there are, and how they differ, has proven difficult to achieve. We suggest that just five structurally distinct types of sensory endings are present in the gut wall that account for essentially all of the primary afferent neurons in the three pathways. Each of these five major structural types of endings seems to show distinctive combinations of physiological responses. These types are: 'intraganglionic laminar' endings in myenteric ganglia; 'mucosal' endings located in the subepithelial layer; 'muscular–mucosal' afferents, with mechanosensitive endings close to the muscularis mucosae; 'intramuscular' endings, with endings within the smooth muscle layers; and 'vascular' afferents, with sensitive endings primarily on blood vessels. 'Silent' afferents might be a subset of inexcitable 'vascular' afferents, which can be switched on by inflammatory mediators. Extrinsic sensory neurons comprise an attractive focus for targeted therapeutic intervention in a range of gastrointestinal disorders.
Key Points
-
The gut is innervated by several classes of extrinsic sensory neurons that have distinct combinations of properties making them sensitive to particular mechanical and chemical stimuli
-
Progress has been made in identifying the morphology of sensory endings in the gut wall, possibly providing a more robust means to classify sensory innervation
-
Five different morphological types of endings can be distinguished by their structure; these account for the great majority of sensory nerves to the gastrointestinal tract and seem to correspond to distinct major physiological classes
-
The physiological properties of extrinsic afferent nerves innervating the gut are characterized by variability and by plasticity, which can make it difficult to reliably distinguish the classes of sensory neurons that underlie gut sensation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Craig, A. D. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 13, 500–505 (2003).
Van Oudenhove, L., Coen, S.-J. & Aziz, Q. Functional brain imaging of gastrointestinal sensation in health and disease. World J. Gastroenterol. 13, 3438–3445 (2007).
Jones, M. P., Dilley, J. B., Drossman, D. & Crowell, M. D. Brain–gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol. Motil. 18, 91–103 (2006).
Melzack, R. & Wall, P. D. Pain mechanisms: a new theory. Science 150, 971–979 (1965).
Mayer, E. A. Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci. 12, 453–466 (2011).
Raybould, H. E. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 153, 41–46 (2010).
Rindi, G., Leiter, A. B., Kopin, A. S., Bordi, C. & Solcia, E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann. N. Y. Acad. Sci. 1014, 1–12 (2004).
Collins, S. M. & Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136, 2003–2014 (2009).
Forsythe, P., Sudo, N., Dinan, T., Taylor, V. H. & Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 24, 9–16 (2010).
Keita, A. V. & Soderholm, J. D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 22, 718–733 (2010).
Jiang, W. et al. 'First-in-man': Characterising the mechanosensitivity of human colonic afferents. Gut 60, 281–282 (2011).
Peiris, M. et al. Human visceral afferent recordings: Preliminary report. Gut 60, 204–208 (2011).
Sirotin, B. Z. Electrophysiological study of reception from certain internal organs in man. Bull. Exp. Biol. Med. 50, 873–877 (1961).
Rinaman, L., Card, J. P., Schwaber, J. S. & Miselis, R. R. Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J. Neurosci. 9, 1985–1996 (1989).
Willis, A., Mihalevich, M., Neff, R. A. & Mendelowitz, D. Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am. J. Physiol. 271, R1614–R1619 (1996).
Zhang, X. & Fogel, R. Involvement of glutamate in gastrointestinal vago-vagal reflexes initiated by gastrointestinal distention in the rat. Auton. Neurosci. 103, 19–37 (2003).
Dembowsky, K., Czachurski, J. & Seller, H. An intracellular study of the synaptic input to sympathetic preganglionic neurones of the third thoracic segment of the cat. J. Auton. Nerv. Syst. 13, 201–244 (1985).
Matthews, M. R. & Cuello, A. C. Substance P-immunoreactive peripheral branches of sensory neurons innervate guinea pig sympathetic neurons. Proc. Natl Acad. Sci. USA 79, 1668–1672 (1982).
De Groat, W. C. & Krier, J. The sacral parasympathetic reflex pathway regulating colonic motility and defaecation in the cat. J. Physiol. Lond. 276, 481–500 (1978).
Iggo, A. Tension receptors in the stomach and the urinary bladder. J. Physiol. 128, 593–607 (1955).
Paintal, A. A study of gastric stretch receptors. Their role in the peripheral mechanism of satiation of hunger and thirst. J. Physiol. 126, 255–270 (1954).
Brookes, S. J. H., Zagorodnyuk, V. P. & Costa, M. in Advances in vagal afferent neurobiology (eds Undem, B. J. & Weinreich, D.) 147–166 (CRC Press, FL, 2005).
Tassicker, B. C., Hennig, G. W., Costa, M. & Brookes, S. J. H. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tissue Res. 295, 437–452 (1999).
Zagorodnyuk, V. P. & Brookes, S. J. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci. 20, 6249–6255 (2000).
Zagorodnyuk, V. P., Chen, B. N. & Brookes, S. J. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 534, 255–268 (2001).
Zagorodnyuk, V. P., Chen, B. N., Costa, M. & Brookes, S. J. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J. Physiol. 553, 575–587 (2003).
Andrews, P. L., Grundy, D. & Scratcherd, T. Vagal afferent discharge from mechanoreceptors in different regions of the ferret stomach. J. Physiol. Lond. 298, 513–524 (1980).
Berthoud, H. R., Patterson, L. M., Neumann, F. & Neuhuber, W. L. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat. Embryol. (Berl.) 195, 183–191 (1997).
Fox, E. A., Phillips, R. J., Martinson, F. A., Baronowsky, E. A. & Powley, T. L. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J. Comp. Neurol. 428, 558–576 (2000).
Berthoud, H. R., Lynn, P. A. & Blackshaw, L. A. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1371–R1381 (2001).
Zhong, F., Christianson, J. A., Davis, B. M. & Bielefeldt, K. Dichotomizing axons in spinal and vagal afferents of the mouse stomach. Dig. Dis. Sci. 53, 194–203 (2008).
Neuhuber, W. L. & Clerc, N. in The Primary Afferent Neuron: A Survey of Recent Morpho-functional Aspects (eds Zenker, W. & Neuhuber, W. L.) 93–107 (Plenum, New York, 1990).
Page, A. J., Martin, C. M. & Blackshaw, L. A. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J. Neurophysiol. 87, 2095–2103 (2002).
Cook, S. P. & McCleskey, E. W. Cell damage excites nociceptors through release of cytosolic ATP. Pain 95, 41–47 (2002).
Cockayne, D. A. et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407, 1011–1015 (2000).
Wynn, G., Rong, W., Xiang, Z. & Burnstock, G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colon. Gastroenterology 125, 1398–1409 (2003).
Smid, S. D., Young, R. L., Cooper, N. J. & Blackshaw, L. A. GABA(B)R expressed on vagal afferent neurones inhibit gastric mechanosensitivity in ferret proximal stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G1494–G1501 (2001).
Page, A. J. et al. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology 128, 402–410 (2005).
Slattery, J. A., Page, A. J., Dorian, C. L., Brierley, S. M. & Blackshaw, L. A. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J. Physiol. 577, 295–306 (2006).
Kentish, S. et al. Diet-induced adaptation of vagal afferent function. J. Physiol. 590, 209–221 (2012).
Olsson, C., Costa, M. & Brookes, S. J. Neurochemical characterization of extrinsic innervation of the guinea pig rectum. J. Comp. Neurol. 470, 357–371 (2004).
Lynn, P. A., Olsson, C., Zagorodnyuk, V., Costa, M. & Brookes, S. J. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 125, 786–794 (2003).
Zagorodnyuk, V. P. et al. Loss of visceral pain following colorectal distension in an endothelin-3 deficient mouse model of hirschsprung's disease. J. Physiol. 589, 1691–1706 (2011).
Lynn, P., Zagorodnyuk, V., Hennig, G., Costa, M. & Brookes, S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J. Physiol. 564, 589–601 (2005).
Janig, W. & Koltzenburg, M. Receptive properties of sacral primary afferent neurons supplying the colon. J. Neurophysiol. 65, 1067–1077 (1991).
Sengupta, J. N. & Gebhart, G. F. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J. Neurophysiol. 71, 2046–2060 (1994).
Brierley, S. M., Jones, R. C. 3rd., Gebhart, G. F. & Blackshaw, L. A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178 (2004).
Yamanouchi, M. et al. Integrative control of rectoanal reflex in guinea pigs through lumbar colonic nerves. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G148–G156 (2002).
Denny-Brown, D. & Robertson, E. An investigation of the nervous control of defaecation. Brain 58, 256–310 (1935).
Paintal, A. S. Responses from mucosal mechanoreceptors in the small intestine of the cat. J. Physiol. 139, 353–368 (1957).
Clarke, G. D. & Davison, J. S. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J. Physiol. Lond. 284, 55–67 (1978).
Leek, B. F. Abdominal and pelvic visceral receptors. Br. Med. Bull. 33, 163–168 (1977).
Powley, T. L. & Phillips, R. J. Vagal intramuscular array afferents form complexes with interstitial cells of cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience 186, 188–200 (2011).
Steinert, R. E. & Beglinger, C. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol. Behav. 105, 62–70 (2011).
Gershon, M. D. & Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007).
Gibbs, J., Young, R. C. & Smith, G. P. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature 245, 323–325 (1973).
Hewson, G., Leighton, G. E., Hill, R. G. & Hughes, J. The cholecystokinin receptor antagonist L364,718 increases food intake in the rat by attenuation of the action of endogenous cholecystokinin. Br. J. Pharmacol. 93, 79–84 (1988).
Smith, G. P., Jerome, C., Cushin, B. J., Eterno, R. & Simansky, K. J. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213, 1036–1037 (1981).
Blackshaw, L. A. & Grundy, D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J. Auton. Nerv. Syst. 31, 191–201 (1990).
Eastwood, C., Maubach, K., Kirkup, A. J. & Grundy, D. The role of endogenous cholecystokinin in the sensory transduction of luminal nutrient signals in the rat jejunum. Neurosci. Lett. 254, 145–148 (1998).
Lal, S., Kirkup, A. J., Brunsden, A. M., Thompson, D. G. & Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G907–G915 (2001).
Abbott, C. R. et al. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 1044, 127–131 (2005).
Koda, S. et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 146, 2369–2375 (2005).
Batterham, R. L. et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418, 650–654 (2002).
Li, Y., Wu, X., Yao, H. & Owyang, C. Secretin activates vagal primary afferent neurons in the rat: evidence from electrophysiological and immunohistochemical studies. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G745–G752 (2005).
Kreis, M. E., Jiang, W., Kirkup, A. J. & Grundy, D. Cosensitivity of vagal mucosal afferents to histamine and 5-HT in the rat jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G612–G617 (2002).
Hillsley, K. & Grundy, D. Serotonin and cholecystokinin activate different populations of rat mesenteric vagal afferents. Neurosci. Lett. 255, 63–66 (1998).
Grundy, D., Blackshaw, L. A. & Hillsley, K. Role of 5-hydroxytryptamine in gastrointestinal chemosensitivity. Dig. Dis. Sci. 39, 44S–47S (1994).
Hicks, G. A. et al. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J. Physiol. 544, 861–869 (2002).
Page, A. J. & Blackshaw, L. A. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J. Physiol. Lond. 512, 907–916 (1998).
Berthoud, H. R. & Powley, T. L. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol. 319, 261–276 (1992).
Wang, F. B. & Powley, T. L. Topographic inventories of vagal afferents in gastrointestinal muscle. J. Comp. Neurol. 421, 302–324 (2000).
Yu, S., Undem, B. J. & Kollarik, M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J. Physiol. 563, 831–842 (2005).
Hayakawa, T., Kuwahara-Otani, S., Maeda, S., Tanaka, K. & Seki, M. Projections of calcitonin gene-related peptide immunoreactive neurons in the vagal ganglia of the rat. J. Chem. Neuroanat. 41, 55–62 (2011).
Spencer, N. J. et al. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience 153, 518–534 (2008).
Spencer, N. J. et al. Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G855–G867 (2008).
Lynn, P. A. & Brookes, S. J. H. Pudendal afferent innervation of the guinea pig external anal sphincter. Neurogastroenterol. Motil. 23, 871–e343 (2011).
Dutsch, M. et al. Vagal and spinal afferent innervation of the rat esophagus: a combined retrograde tracing and immunocytochemical study with special emphasis on calcium-binding proteins. J. Comp. Neurol. 398, 289–307 (1998).
Sang, Q. & Young, H. M. The origin and development of the vagal and spinal innervation of the external muscle of the mouse esophagus. Brain Res. 809, 253–268 (1998).
Bessou, P. & Perl, E. R. A movement receptor of the small intestine. J. Physiol. 182, 404–426 (1966).
Feng, B., Brumovsky, P. R. & Gebhart, G. F. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G402–G409 (2010).
Feng, B. et al. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G676–G683 (2012).
Blumberg, H., Haupt, P., Janig, W. & Kohler, W. Encoding of visceral noxious stimuli in the discharge patterns of visceral afferent fibres from the colon. Pflugers Arch. 398, 33–40 (1983).
Cottrell, D. F. Mechanoreceptors of the rabbit duodenum. Q. J. Exp. Physiol. 69, 677–684 (1984).
Morrison, J. F. Splanchnic slowly adapting mechanoreceptors with punctate receptive fields in the mesentery and gastrointestinal tract of the cat. J. Physiol. 233, 349–361 (1973).
Floyd, K. & Morrison, J. F. Splanchnic mechanoreceptors in the dog. Q. J. Exp. Physiol. Cogn. Med. Sci. 59, 361–366 (1974).
Haupt, P., Janig, W. & Kohler, W. Response pattern of visceral afferent fibres, supplying the colon, upon chemical and mechanical stimuli. Pflugers Arch. 398, 41–47 (1983).
Longhurst, J. C. & Dittman, L. E. Hypoxia, bradykinin, and prostaglandins stimulate ischemically sensitive visceral afferents. Am. J. Physiol. 253, H556–H567 (1987).
Longhurst, J. C., Kaufman, M. P., Ordway, G. A. & Musch, T. I. Effects of bradykinin and capsaicin on endings of afferent fibers from abdominal visceral organs. Am. J. Physiol. 247, R552–R559 (1984).
Brunsden, A. M., Jacob, S., Bardhan, K. D. & Grundy, D. Mesenteric afferent nerves are sensitive to vascular perfusion in a novel preparation of rat ileum in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G656–G665 (2002).
Brunsden, A. M., Brookes, S. J., Bardhan, K. D. & Grundy, D. Mechanisms underlying mechanosensitivity of mesenteric afferent fibers to vascular flow. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G422–G428 (2007).
Malin, S. A., Christianson, J. A., Bielefeldt, K. & Davis, B. M. TPRV1 expression defines functionally distinct pelvic colon afferents. J. Neurosci. 29, 743–752 (2009).
Song, X. et al. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 137, 274–284 (2009).
Takaki, M. & Nakayama, S. Effects of capsaicin on myenteric neurons of the guinea pig ileum. Neurosci. Lett. 105, 125–130 (1989).
Takaki, M. & Nakayama, S. Electrical behavior of myenteric neurons induced by mesenteric nerve stimulation in the guinea pig ileum. Acta Med. Okayama 44, 257–261 (1990).
Bartho, L., Holzer, P., Lembeck, F. & Szolcsanyi, J. Evidence that the contractile response of the guinea-pig ileum to capsaicin is due to release of substance P. J. Physiol. Lond. 332, 157–167 (1982).
Holzer, P. in Physiology of the Gastrointestinal Tract Vol. 1 (ed. Johnson, L. R.) 817–845 (Elsevier, Amsterdam, 2012).
Bayliss, W. M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J. Physiol. 26, 173–209 (1901).
Gibbins, I. L. et al. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci. Lett. 57, 125–130 (1985).
Meehan, A. G., Hottenstein, O. D. & Kreulen, D. L. Capsaicin-sensitive nerves mediate inhibitory junction potentials and dilatation in guinea-pig mesenteric artery. J. Physiol. 443, 161–174 (1991).
Uddman, R., Edvinsson, L., Ekblad, E., Hakanson, R. & Sundler, F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul. Pept. 15, 1–23 (1986).
Kawasaki, H., Takasaki, K., Saito, A. & Goto, K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 335, 164–167 (1988).
Vanner, S. & Surprenant, A. Neural reflexes controlling intestinal microcirculation. Am. J. Physiol. 271, G223–G230 (1996).
Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 30, 5–11 (1998).
Figini, M. et al. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am. J. Physiol. 272, G785–G793 (1997).
Sann, H., Dux, M., Schemann, M. & Jancso, G. Neurogenic inflammation in the gastrointestinal tract of the rat. Neurosci. Lett. 219, 147–150 (1996).
Meehan, A. G. & Kreulen, D. L. A capsaicin-sensitive inhibitory reflex from the colon to mesenteric arteries in the guinea-pig. J. Physiol. Lond. 448, 153–159 (1992).
Petersen, K. A. et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia 25, 139–147 (2005).
Blackshaw, L. A. & Gebhart, G. F. The pharmacology of gastrointestinal nociceptive pathways. Curr. Opin. Pharmacol. 2, 642–649 (2002).
Binshtok, A. M. et al. Nociceptors are interleukin-1beta sensors. J. Neurosci. 28, 14062–14073 (2008).
Andratsch, M. et al. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J. Neurosci. 29, 13473–13483 (2009).
Li, Y., Ji, A., Weihe, E. & Schafer, M. K. H. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J. Neurosci. 24, 9623–9631 (2004).
Hughes, P. A. et al. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58, 1333–1341 (2009).
Sengupta, J. N., Snider, A., Su, X. & Gebhart, G. F. Effects of kappa opioids in the inflamed rat colon. Pain 79, 175–185 (1999).
Gschossmann, J. M. et al. Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig. Dis. Sci. 49, 96–101 (2004).
Brierley, S. M. et al. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J. Physiol. 567, 267–281 (2005).
Robinson, D. R., McNaughton, P. A., Evans, M. L. & Hicks, G. A. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol. Motil. 16, 113–124 (2004).
Ward, S. M., Bayguinov, J., Won, K. J., Grundy, D. & Berthoud, H. R. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J. Comp. Neurol. 465, 121–135 (2003).
Woo, Y. C., Park, S. S., Subieta, A. R. & Brennan, T. J. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 101, 468–475 (2004).
Cho, H. et al. The calcium-activated chloride channel anoctamin-1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 15, 1015–1021 (2012).
Yiangou, Y. et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 357, 1338–1339 (2001).
Akbar, A. et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57, 923–929 (2008).
De Schepper, H. U. et al. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J. Physiol. 586, 5247–5258 (2008).
Brierley, S. M. et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134, 2059–2069 (2008).
Nilius, B., Vriens, J., Prenen, J., Droogmans, G. & Voets, T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am. J. Physiol. Cell Physiol. 286, C195–C205 (2004).
Cenac, N. et al. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135, 937–946 (2008).
Steinhoff, M. et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 6, 151–158 (2000).
Sipe, W. E. B. et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1288–G1298 (2008).
Cattaruzza, F. et al. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G81–G91 (2010).
Brierley, S. M. et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137, 2084–2095 (2009).
Nilius, B., Prenen, J. & Owsianik, G. Irritating channels: The case of TRPA1. J. Physiol. 589, 1543–1549 (2011).
Miyamoto, R., Otsuguro, K.-I. & Ito, S. Time- and concentration-dependent activation of TRPA1 by hydrogen sulfide in rat drg neurons. Neurosci. Lett. 499, 137–142 (2011).
Feng, B. & Gebhart, G. F. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G170–G180 (2011).
Olsson, C. et al. Comparison of extrinsic efferent innervation of guinea pig distal colon and rectum. J. Comp. Neurol. 496, 787–801 (2006).
Lynn, P. A., Chen, B. N., Zagorodnyuk, V. P., Costa, M. & Brookes, S. J. H. TNBS-induced inflammation modulates the function of one class of low-threshold rectal mechanoreceptors in the guinea pig. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G862–G871 (2008).
Beyak, M. J. Visceral afferents—determinants and modulation of excitability. Auton. Neurosci. 153, 69–78 (2010).
Furness, J. B. Novel gut afferents: Intrinsic afferent neurons and intestinofugal neurons. Auton. Neurosci. 125, 81–85 (2006).
Blackshaw, L. A., Brookes, S. J., Grundy, D. & Schemann, M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol. Motil. 19, 1–19 (2007).
Doerffler-Melly, J. & Neuhuber, W. L. Rectospinal neurons: Evidence for a direct projection from the enteric to the central nervous system in the rat. Neurosci. Lett. 92, 121–125 (1988).
Neuhuber, W. L. et al. Rectospinal neurons: cell bodies, pathways, immunocytochemistry and ultrastructure. Neuroscience 56, 367–378 (1993).
Suckow, S. K. & Caudle, R. M. Identification and immunohistochemical characterization of colospinal afferent neurons in the rat. Neuroscience 153, 803–813 (2008).
Hibberd, T. J., Zagorodnyuk, V. P., Spencer, N. J. & Brookes, S. J. H. Viscerofugal neurons recorded from guinea-pig colonic nerves after organ culture. Neurogastroenterol. Motil. 24, 1041–e548 (2012).
Hibberd, T. J., Zagorodnyuk, V. P., Spencer, N. J. & Brookes, S. J. H. Identification and mechanosensitivity of viscerofugal neurons. Neuroscience 225, 118–129 (2012).
Sharkey, K. A., Lomax, A. E., Bertrand, P. P. & Furness, J. B. Electrophysiology, shape, and chemistry of neurons that project from guinea pig colon to inferior mesenteric ganglia. Gastroenterology 115, 909–918 (1998).
Ness, T. J. & Gebhart, G. F. Visceral pain: a review of experimental studies. Pain 41, 167–234 (1990).
Kyloh, M., Nicholas, S., Zagorodnyuk, V. P., Brookes, S. J. H. & Spencer, N. J. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front. Neurosci. 22, 11–17 (2011).
Traub, R. J. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11, 2113–2116 (2000).
Ray, B. S. & Neill, C. L. Abdominal visceral sensation in man. Ann. Surg. 126, 709–724 (1947).
Lembo, T. et al. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology 107, 1686–1696 (1994).
Rao, S. S. C. Pathophysiology of adult fecal incontinence. Gastroenterology 126, S14–S22 (2004).
Kwan, C. L., Mikula, K., Diamant, N. E. & Davis, K. D. The relationship between rectal pain, unpleasantness, and urge to defecate in normal subjects. Pain 97, 53–63 (2002).
Powley, T. L. & Phillips, R. J. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 82, 69–74 (2004).
Feinle, C., Grundy, D. & Read, N. W. Effects of duodenal nutrients on sensory and motor responses of the human stomach to distension. Am. J. Physiol. 273, G721–G726 (1997).
Andrews, P. L. R. & Horn, C. C. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton. Neurosci. 125, 100–115 (2006).
Sanger, G. J. & Andrews, P. L. R. Treatment of nausea and vomiting: gaps in our knowledge. Auton. Neurosci. 129, 3–16 (2006).
Zhu, J. X., Zhu, X. Y., Owyang, C. & Li, Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J. Physiol. 530, 431–442 (2001).
Carmagnola, S., Cantu, P. & Penagini, R. Mechanoreceptors of the proximal stomach and perception of gastric distension. Am. J. Gastroenterol. 100, 1704–1710 (2005).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Brookes, S., Spencer, N., Costa, M. et al. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol 10, 286–296 (2013). https://doi.org/10.1038/nrgastro.2013.29
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.29
This article is cited by
-
Butterflies in the gut: the interplay between intestinal microbiota and stress
Journal of Biomedical Science (2023)
-
Systemic sclerosis gastrointestinal dysmotility: risk factors, pathophysiology, diagnosis and management
Nature Reviews Rheumatology (2023)
-
Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits
Nature Biotechnology (2023)
-
Spinal afferent innervation in flat-mounts of the rat stomach: anterograde tracing
Scientific Reports (2023)
-
Features of the Neurophysiological Mechanisms of Visceral and Somatic Pain
Neuroscience and Behavioral Physiology (2023)