Key Points
-
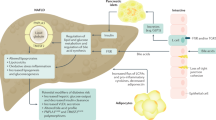
As a result of increased prevalence, NAFLD and type 2 diabetes mellitus (T2DM) are of increasing clinical relevance worldwide
-
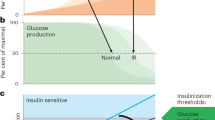
The liver has a key role in the pathophysiology of T2DM, as this organ contributes substantially to the development of insulin resistance
-
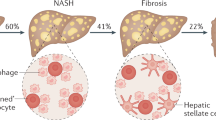
Rates of NAFLD, NASH, liver cirrhosis and hepatocellular carcinoma in patients with T2DM are rapidly rising
-
Medications used in the treatment of T2DM are also effective in the therapy of NASH
Abstract
The liver constitutes a key organ in systemic metabolism, contributing substantially to the development of insulin resistance and type 2 diabetes mellitus (T2DM). The mechanisms underlying these processes are not entirely understood, but involve hepatic fat accumulation, alterations of energy metabolism and inflammatory signals derived from various cell types including immune cells. Lipotoxins, mitochondrial function, cytokines and adipocytokines have been proposed to play a major part in both NAFLD and T2DM. Patients with NAFLD are commonly insulin resistant. On the other hand, a large number of patients with T2DM develop NAFLD with its inflammatory complication, NASH. The high incidence of NASH in patients with T2DM leads to further complications, such as liver cirrhosis and hepatocellular carcinoma, which are increasingly recognized. Therapeutic concepts such as thiazolidinediones (glitazones) for treating T2DM also show some efficacy in the treatment of NASH. This Review will describe the multifaceted and complex interactions between the liver and T2DM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ratziu, V., Goodman, Z. & Sanyal, A. Current efforts and trends in the treatment of NASH. J. Hepatol. 62, S65–75 (2015).
Ahmed, A., Wong, R. J. & Harrison, S. A. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin. Gastroenterol. Hepatol. 13, 2062–2070 (2015).
Castera, L., Vilgrain, V. & Angulo, P. Noninvasive evaluation of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 10, 666–675 (2013).
Hyysalo, J. et al. A population-based study on the prevalence of NASH using scores validated against liver histology. J. Hepatol. 60, 839–846 (2014).
Yeh, M. M. & Brunt, E. M. Pathological features of fatty liver disease. Gastroenterology 147, 754–764 (2014).
Wree, A., Broderick, L., Canbay, A., Hoffman, H. M. & Feldstein, A. E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 10, 627–636 (2013).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Marchesini, G. et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 107, 450–455 (1999).
Haas, J. T., Francque, S. & Staels, B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu. Rev. Physiol. 78, 181–205 (2016).
Moschen, A. R., Kaser, S. & Tilg, H. Non-alcoholic steatohepatitis: a microbiota-driven disease. Trends Endocrinol. Metab. 24, 537–545 (2013).
Williams, C. D. et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140, 124–131 (2011).
Loomba, R. et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 56, 943–951 (2012).
Doycheva, I., Patel, N., Peterson, M. & Loomba, R. Prognostic implication of liver histology in patients with nonalcoholic fatty liver disease in diabetes. J. Diabetes Complications 27, 293–300 (2013).
Kwok, R. et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 65, 1359–1368 (2015).
Koehler, E. M. et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 63, 138–147 (2016).
Collaboration, N. C. D. R. F. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530 (2016).
Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2, 901–910 (2014).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 (2013).
Lonardo, A., Ballestri, S., Marchesini, G., Angulo, P. & Loria, P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig. Liver Dis. 47, 181–190 (2015).
Roden, M. Mechanisms of disease: hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2, 335–348 (2006).
Kotronen, A., Westerbacka, J., Bergholm, R., Pietilainen, K. H. & Yki-Jarvinen, H. Liver fat in the metabolic syndrome. J. Clin. Endocrinol. Metab. 92, 3490–3497 (2007).
Gaggini, M. et al. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 5, 1544–1560 (2013).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155 (2015).
Krssak, M. et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53, 3048–3056 (2004).
Lomonaco, R. et al. Metabolic impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care 39, 632–638 (2016).
Marchesini, G. et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50, 1844–1850 (2001).
Utzschneider, K. M. et al. Insulin resistance is the best predictor of the metabolic syndrome in subjects with a first-degree relative with type 2 diabetes. Obesity (Silver Spring) 18, 1781–1787 (2010).
Iozzo, P. et al. Defective liver disposal of free fatty acids in patients with impaired glucose tolerance. J. Clin. Endocrinol. Metab. 89, 3496–3502 (2004).
Nielsen, S., Guo, Z., Johnson, C. M., Hensrud, D. D. & Jensen, M. D. Splanchnic lipolysis in human obesity. J. Clin. Invest. 113, 1582–1588 (2004).
Donnelly, K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115, 1343–1351 (2005).
Sanyal, A. J. et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120, 1183–1192 (2001).
Lomonaco, R. et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55, 1389–1397 (2012).
Fabbrini, E. et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 134, 424–431 (2008).
Lambert, J. E., Ramos-Roman, M. A., Browning, J. D. & Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735 (2014).
Petersen, K. F. et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 104, 12587–12594 (2007).
Roden, M. et al. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 49, 701–707 (2000).
Shulman, G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 2237–2238 (2014).
Szendroedi, J. et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 111, 9597–9602 (2014).
Samuel, V. T. et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 (2004).
Kumashiro, N. et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 108, 16381–16385 (2011).
Magkos, F. et al. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 142, 1444–1446.e2 (2012).
Unger, R. H. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 14, 398–403 (2003).
Szendroedi, J. et al. Lower fasting muscle mitochondrial activity relates to hepatic steatosis in humans. Diabetes Care 37, 468–474 (2014).
Szendroedi, J. et al. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 50, 1079–1086 (2009).
Schmid, A. I. et al. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care 34, 448–453 (2011).
Schmid, A. I. et al. Quantitative ATP synthesis in human liver measured by localized 31P spectroscopy using the magnetization transfer experiment. NMR Biomed. 21, 437–443 (2008).
Koliaki, C. et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 21, 739–746 (2015).
Fritsch, M. et al. Time course of postprandial hepatic phosphorus metabolites in lean, obese, and type 2 diabetes patients. Am. J. Clin. Nutr. 102, 1051–1058 (2015).
Cortez-Pinto, H. et al. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA 282, 1659–1664 (1999).
Satapati, S. et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Invest. 125, 4447–4462 (2015).
Perez-Carreras, M. et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38, 999–1007 (2003).
Serviddio, G. et al. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut 57, 957–965 (2008).
Morris, E. M., Rector, R. S., Thyfault, J. P. & Ibdah, J. A. Mitochondria and redox signaling in steatohepatitis. Antioxid. Redox Signal. 15, 485–504 (2011).
Romestaing, C. et al. Mitochondrial adaptations to steatohepatitis induced by a methionine- and choline-deficient diet. Am. J. Physiol. Endocrinol. Metab. 294, E110–E119 (2008).
Titchenell, P. M., Chu, Q., Monks, B. R. & Birnbaum, M. J. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat. Commun. 6, 7078 (2015).
Haeusler, R. A. et al. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat. Commun. 5, 5190 (2014).
Lu, M. et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 18, 388–395 (2012).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
He, S. et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 285, 6706–6715 (2010).
Donati, B. et al. The rs2294918 E434K variant modulates patatin-like phospholipase domain-containing 3 expression and liver damage. Hepatology 63, 787–798 (2016).
Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356 (2014).
Zhou, Y. et al. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. J. Hepatol. 62, 657–663 (2015).
Lee, H. Y. et al. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology 54, 1650–1660 (2011).
Amaro, A. et al. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology 139, 149–153 (2010).
Tilg, H. & Moschen, A. R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 14, 222–231 (2008).
Johnson, A. M. & Olefsky, J. M. The origins and drivers of insulin resistance. Cell 152, 673–684 (2013).
Moller, D. E. & Kaufman, K. D. Metabolic syndrome: a clinical and molecular perspective. Annu. Rev. Med. 56, 45–62 (2005).
Morris, E. M., Rector, R. S., Thyfault, J. P. & Ibdah, J. A. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin Invest. 94, 1543–1549 (1994).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012).
Wieser, V., Moschen, A. R. & Tilg, H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch. Immunol. Ther. Exp. (Warsz) 61, 119–125 (2013).
Tilg, H. & Diehl, A. M. Cytokines in alcoholic and nonalcoholic steatohepatitis. N. Engl. J. Med. 343, 1467–1476 (2000).
Le, K. A. et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes 60, 2802–2809 (2011).
Yuan, M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293, 1673–1677 (2001).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Kiechl, S. et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat. Med. 19, 358–363 (2013).
Kong, Y. Y. et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402, 304–309 (1999).
Goto, H. et al. Primary human bone marrow adipocytes support TNF-α-induced osteoclast differentiation and function through RANKL expression. Cytokine 56, 662–668 (2011).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 (2007).
Moschen, A. R. et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor α expression. Gut 59, 1259–1264 (2010).
Moschen, A. R. et al. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 17, 840–845 (2011).
Cao, H. et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17, 768–778 (2013).
Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52, 1836–1846 (2010).
Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab. 17, 851–859 (2013).
Okada-Iwabu, M. et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503, 493–499 (2013).
Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346, 1221–1231 (2002).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Lee, D. H. et al. Gamma-glutamyltransferase and diabetes—a 4 year follow-up study. Diabetologia 46, 359–364 (2003).
Hanley, A. J. et al. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes 54, 3140–3147 (2005).
Goessling, W. et al. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 135, 1935–1944.e1 (2008).
Fraser, A. et al. Alanine aminotransferase, γ-glutamyltransferase, and incident diabetes: the British Women's Heart and Health Study and meta-analysis. Diabetes Care 32, 741–750 (2009).
Fracanzani, A. L. et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 48, 792–798 (2008).
Armstrong, M. J., Adams, L. A., Canbay, A. & Syn, W. K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 59, 1174–1197 (2014).
Sung, K. C., Wild, S. H. & Byrne, C. D. Resolution of fatty liver and risk of incident diabetes. J. Clin. Endocrinol. Metab. 98, 3637–3643 (2013).
Pais, R. et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 59, 550–556 (2013).
Ekstedt, M. et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44, 865–873 (2006).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 59, 1121–1240 (2016).
Portillo-Sanchez, P. et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J. Clin. Endocrinol. Metab. 100, 2231–2238 (2015).
Poonawala, A., Nair, S. P. & Thuluvath, P. J. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology 32, 689–692 (2000).
Ghouri, N., Preiss, D. & Sattar, N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology 52, 1156–1161 (2010).
Leite, N. C., Salles, G. F., Araujo, A. L., Villela-Nogueira, C. A. & Cardoso, C. R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 29, 113–119 (2009).
Doycheva, I. et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment. Pharmacol. Ther. 43, 83–95 (2016).
Abrams, G. A., Kunde, S. S., Lazenby, A. J. & Clements, R. H. Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of nonalcoholic fatty liver disease. Hepatology 40, 475–483 (2004).
Goh, G. B. et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 3, 141–145 (2015).
Bazick, J. et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care 38, 1347–1355 (2015).
Morling, J. R. et al. Using non-invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: the Edinburgh type 2 diabetes study. J. Hepatol. 60, 384–391 (2014).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397.e10 (2015).
Angulo, P., Keach, J. C., Batts, K. P. & Lindor, K. D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 30, 1356–1362 (1999).
Hossain, N. et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1224–1229, 1229.e1–1229.e2 (2009).
Adams, L. A. et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am. J. Gastroenterol. 105, 1567–1573 (2010).
Targher, G. et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30, 1212–1218 (2007).
Targher, G. et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 51, 444–450 (2008).
Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol. 62 (Suppl. 1), S47–S64 (2015).
Marengo, A., Rosso, C. & Bugianesi, E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 67, 103–117 (2016).
Bugianesi, E. et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 123, 134–140 (2002).
Oberaigner, W. et al. Increased cancer incidence risk in type 2 diabetes mellitus: results from a cohort study in Tyrol/Austria. BMC Public Health 14, 1058 (2014).
Dyson, J. et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 60, 110–117 (2014).
Zheng, Z. et al. Diabetes mellitus is associated with hepatocellular carcinoma: a retrospective case-control study in hepatitis endemic area. PLoS ONE 8, e84776 (2013).
Yasui, K. et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 9, 428–433 (2011).
Welzel, T. M. et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am. J. Gastroenterol. 108, 1314–1321 (2013).
Aleksandrova, K. et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology 60, 858–871 (2014).
Chiang, C. H. et al. The relationship of diabetes and smoking status to hepatocellular carcinoma mortality. Medicine (Baltimore) 95, e2699 (2016).
Raff, E. J. et al. Diabetes mellitus predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases. J. Clin. Transl Hepatol. 3, 9–16 (2015).
Musso, G., Cassader, M., Rosina, F. & Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 55, 885–904 (2012).
Phielix, E., Szendroedi, J. & Roden, M. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact. Trends Pharmacol. Sci. 32, 607–616 (2011).
Bugianesi, E. et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 100, 1082–1090 (2005).
Haukeland, J. W. et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand. J. Gastroenterol. 44, 853–860 (2009).
Shields, W. W., Thompson, K. E., Grice, G. A., Harrison, S. A. & Coyle, W. J. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Therap. Adv. Gastroenterol. 2, 157–163 (2009).
Lavine, J. E. et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 305, 1659–1668 (2011).
Torres, D. M. et al. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology 54, 1631–1639 (2011).
Franciosi, M. et al. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS ONE 8, e71583 (2013).
Phielix, E. et al. Effects of pioglitazone versus glimepiride exposure on hepatocellular fat content in type 2 diabetes. Diabetes Obes. Metab. 15, 915–922 (2013).
Belfort, R. et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 355, 2297–2307 (2006).
Aithal, G. P. et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135, 1176–1184 (2008).
Cusi, K. et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann. Intern. Med. 165, 305–315 (2016).
Ratziu, V. et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135, 100–110 (2008).
Ratziu, V. et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51, 445–453 (2010).
Fruci, B., Giuliano, S., Mazza, A., Malaguarnera, R. & Belfiore, A. Nonalcoholic fatty liver: a possible new target for type 2 diabetes prevention and treatment. Int. J. Mol. Sci. 14, 22933–22966 (2013).
Cui, J. et al. Sitagliptin versus placebo for nonalcoholic fatty liver disease: a randomized controlled trial. J. Hepatol. 65, 369–376 (2016).
Singh, S., Khera, R., Allen, A. M., Murad, M. H. & Loomba, R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology 62, 1417–1432 (2015).
Stefan, N. et al. Inhibition of 11β-HSD1 with RO5093151 for non-alcoholic fatty liver disease: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2, 406–416 (2014).
Cariou, B. & Staels, B. GFT505 for the treatment of nonalcoholic steatohepatitis and type 2 diabetes. Expert Opin. Investig. Drugs 23, 1441–1448 (2014).
Ratziu, V. et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150, 1147–1159 (2016).
Perry, R. J., Zhang, D., Zhang, X. M., Boyer, J. L. & Shulman, G. I. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347, 1253–1256 (2015).
Caiazzo, R. et al. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann. Surg. 260, 893–898; discussion 898–899 (2014).
Lassailly, G. et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 149, 379–388; quiz e15–e16 (2015).
Thaler, H. [Fatty liver, its causes and concomitant diseases]. Dtsch Med. Wochenschr. 87, 1049–1055 (in German) (1962).
Author information
Authors and Affiliations
Contributions
H.T. and M.R. researched data for the article. A.R.M., H.T. and M.R. contributed to discussion of content, and wrote, reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tilg, H., Moschen, A. & Roden, M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 14, 32–42 (2017). https://doi.org/10.1038/nrgastro.2016.147
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.147
This article is cited by
-
Phosphorylation: new star of pathogenesis and treatment in steatotic liver disease
Lipids in Health and Disease (2024)
-
Arachidonic acid inhibit granulosa cell function by affecting metabolic function of liver in brown adipose transplantation rats
Journal of Ovarian Research (2024)
-
Dietary intervention reverses molecular markers of hepatocellular senescence in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH
BMC Gastroenterology (2024)
-
The gasdermin family: emerging therapeutic targets in diseases
Signal Transduction and Targeted Therapy (2024)
-
Inter-organ crosstalk during development and progression of type 2 diabetes mellitus
Nature Reviews Endocrinology (2024)