Key Points
-
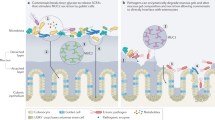
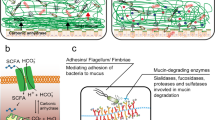
The intestinal epithelium is a dynamic cellular layer that serves as a barrier between luminal contents and the underlying immune system while simultaneously supporting water, nutrient and ion transport
-
Tight junctions are the primary determinants of barrier function in intact epithelia and are composed of a complex network of transmembrane and cytosolic proteins accompanied by cytoskeletal and regulatory proteins
-
Two distinct pathways — termed pore and leak — regulate paracellular flux in intact epithelia whereas the unrestricted flux pathway is the dominant route across ulcerated or denuded epithelia
-
Reduced intestinal epithelial barrier function is associated with a variety of gastrointestinal and systemic diseases, including IBD and graft versus host disease, respectively, but is insufficient to cause disease in the absence of other insults
-
Experimental evidence suggests that barrier defects contribute to IBD, as mouse models demonstrate that increased paracellular permeability accelerates experimental colitis and that preservation of tight junction barrier function delays disease progression
-
Although no currently available therapeutics specifically modulate epithelial barrier function, promising approaches to target the pore, leak, and unrestricted pathways are being investigated
Abstract
A fundamental function of the intestinal epithelium is to act as a barrier that limits interactions between luminal contents such as the intestinal microbiota, the underlying immune system and the remainder of the body, while supporting vectorial transport of nutrients, water and waste products. Epithelial barrier function requires a contiguous layer of cells as well as the junctions that seal the paracellular space between epithelial cells. Compromised intestinal barrier function has been associated with a number of disease states, both intestinal and systemic. Unfortunately, most current clinical data are correlative, making it difficult to separate cause from effect in interpreting the importance of barrier loss. Some data from experimental animal models suggest that compromised epithelial integrity might have a pathogenic role in specific gastrointestinal diseases, but no FDA-approved agents that target the epithelial barrier are presently available. To develop such therapies, a deeper understanding of both disease pathogenesis and mechanisms of barrier regulation must be reached. Here, we review and discuss mechanisms of intestinal barrier loss and the role of intestinal epithelial barrier function in pathogenesis of both intestinal and systemic diseases. We conclude with a discussion of potential strategies to restore the epithelial barrier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Marchiando, A. M., Graham, W. V. & Turner, J. R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 5, 119–144 (2010).
Turner, J. R. in Yamada's Textbook of Gastroenterology (eds Podolsky, D. K. et al.) 317–329 (Wiley-Blackwell, 2015).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Fu, J. et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 121, 1657–1666 (2011).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Johansson, M. E. et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Shen, L., Weber, C. R., Raleigh, D. R., Yu, D. & Turner, J. R. Tight junction pore and leak pathways: a dynamic duo. Annu. Rev. Physiol. 73, 283–309 (2011).
Farquhar, M. & Palade, G. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963).
Madara, J. L. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am. J. Physiol. 253, C171–C175 (1987).
Mooseker, M. S. et al. Brush border cytoskeleton and integration of cellular functions. J. Cell Biol. 99, 104s–112s (1984).
Takeichi, M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 15, 397–410 (2014).
Hartsock, A. & Nelson, W. J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 (2008).
Drees, F., Pokutta, S., Yamada, S., Nelson, W. J. & Weis, W. I. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123, 903–915 (2005).
Maiden, S. L. & Hardin, J. The secret life of α-catenin: moonlighting in morphogenesis. J. Cell Biol. 195, 543–552 (2011).
Capaldo, C. T. & Macara, I. G. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 18, 189–200 (2007).
Maiers, J. L., Peng, X., Fanning, A. S. & DeMali, K. A. ZO-1 recruitment to α-catenin — a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J. Cell Sci. 126, 3904–3915 (2013).
Goodenough, D. A. & Revel, J. P. A fine structural analysis of intercellular junctions in the mouse liver. J. Cell Biol. 45, 272–290 (1970).
Kachar, B. & Reese, T. S. Evidence for the lipidic nature of tight junction strands. Nature 296, 464–466 (1982).
Lingaraju, A. et al. Conceptual barriers to understanding physical barriers. Semin. Cell Dev. Biol. 42, 13–21 (2015).
Furuse, M. et al. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777–1788 (1993).
Stankewich, M. C., Francis, S. A., Vu, Q. U., Schneeberger, E. E. & Lynch, R. D. Alterations in cell cholesterol content modulate Ca2+-induced tight junction assembly by MDCK cells. Lipids 31, 817–828 (1996).
Francis, S. A. et al. Rapid reduction of MDCK cell cholesterol by methyl-β-cyclodextrin alters steady state transepithelial electrical resistance. Eur. J. Cell Biol. 78, 473–484 (1999).
Shen, L. et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 119, 2095–2106 (2006).
Van Itallie, C. M. & Anderson, J. M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403–429 (2006).
Furuse, M., Furuse, K., Sasaki, H. & Tsukita, S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 153, 263–272 (2001).
Amasheh, S. et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115, 4969–4976 (2002).
Weber, C. R. et al. Claudin-2-dependent paracellular channels are dynamically gated. eLife 4, e09906 (2015).
Weber, C. R. et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J. Biol. Chem. 285, 12037–12046 (2010).
Raleigh, D. R. et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J. Cell Biol. 193, 565–582 (2011).
Wada, M., Tamura, A., Takahashi, N. & Tsukita, S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 144, 369–380 (2013).
Tamura, A. et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 140, 913–923 (2011).
Turner, J. R., Buschmann, M. M., Romero-Calvo, I., Sailer, A. & Shen, L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin. Cell Dev. Biol. 36, 204–212 (2014).
Raleigh, D. R. et al. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell 21, 1200–1213 (2010).
Furuse, M. et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 127, 1617–1626 (1994).
Cording, J. et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J. Cell Sci. 126, 554–564 (2013).
Fanning, A. S. & Anderson, J. M. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci. 1165, 113–120 (2009).
Anderson, J. M., Fanning, A. S., Lapierre, L. & Van Itallie, C. M. Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem. Soc. Trans. 23, 470–475 (1995).
Katsuno, T. et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol. Biol. Cell 19, 2465–2475 (2008).
Xu, J. et al. Early embryonic lethality of mice lacking ZO-2, but not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol. Cell. Biol. 28, 1669–1678 (2008).
Carlton, V. E. et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat. Genet. 34, 91–96 (2003).
Sambrotta, M. et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat. Genet. 46, 326–328 (2014).
Matsumoto, K. et al. Claudin 2 deficiency reduces bile flow and increases susceptibility to cholesterol gallstone disease in mice. Gastroenterology 147, 1134–1145.e10 (2014).
Luther, J. et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell. Mol. Gastroenterol. Hepatol. 1, 222–232 (2015).
Llorente, C. & Schnabl, B. The gut microbiota and liver disease. Cell. Mol. Gastroenterol. Hepatol. 1, 275–284 (2015).
Van Itallie, C. M., Fanning, A. S., Bridges, A. & Anderson, J. M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 20, 3930–3940 (2009).
Nalle, S. C. & Turner, J. R. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 8, 720–730 (2015).
Su, L. et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 145, 407–415 (2013).
Kiesler, P., Fuss, I. J. & Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 1, 154–170 (2015).
Gitter, A. H., Wullstein, F., Fromm, M. & Schulzke, J. D. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology 121, 1320–1328 (2001).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
van der Flier, L. G. & Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 (2009).
Aoki, R. et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol. 2, 175–188 (2016).
Russo, J. M. et al. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology 128, 987–1001 (2005).
Marchiando, A. M. et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 140, 1208–1218.e2 (2011).
Rosenblatt, J., Raff, M. C. & Cramer, L. P. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847–1857 (2001).
Madara, J. L. & Pappenheimer, J. R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol. 100, 149–164 (1987).
Pappenheimer, J. R. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J. Membr. Biol. 100, 137–148 (1987).
Pappenheimer, J. R. & Reiss, K. Z. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J. Membr. Biol. 100, 123–136 (1987).
Turner, J. R. et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 273, C1378–C1385 (1997).
Turner, J. R. Show me the pathway! Regulation of paracellular permeability by Na+-glucose cotransport. Adv. Drug Deliv. Rev. 41, 265–281 (2000).
Meddings, J. B. & Westergaard, H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin. Sci. (Lond.) 76, 403–413 (1989).
Sadowski, D. C. & Meddings, J. B. Luminal nutrients alter tight-junction permeability in the rat jejunum: an in vivo perfusion model. Can. J. Physiol. Pharmacol. 71, 835–839 (1993).
Turner, J. R., Cohen, D. E., Mrsny, R. J. & Madara, J. L. Noninvasive in vivo analysis of human small intestinal paracellular absorption: regulation by Na+-glucose cotransport. Dig. Dis. Sci. 45, 2122–2126 (2000).
Heller, F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129, 550–564 (2005).
Suzuki, T., Yoshinaga, N. & Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 286, 31263–31271 (2011).
Wisner, D. M., Harris, L. R. III, Green, C. L. & Poritz, L. S. Opposing regulation of the tight junction protein claudin-2 by interferon-γ and interleukin-4. J. Surg. Res. 144, 1–7 (2008).
Mankertz, J. et al. TNFα up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol–3-kinase signaling. Cell Tissue Res. 336, 67–77 (2009).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 (2014).
Zeissig, S. et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56, 61–72 (2007).
Prasad, S. et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Invest. 85, 1139–1162 (2005).
Yu, A. S. et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J. Gen. Physiol. 133, 111–127 (2009).
Li, J., Zhuo, M., Pei, L. & Yu, A. S. Conserved aromatic residue confers cation selectivity in claudin-2 and claudin-10b. J. Biol. Chem. 288, 22790–22797 (2013).
Li, J., Zhuo, M., Pei, L., Rajagopal, M. & Yu, A. S. Comprehensive cysteine-scanning mutagenesis reveals claudin-2 pore-lining residues with different intrapore locations. J. Biol. Chem. 289, 6475–6484 (2014).
Marcial, M. A., Carlson, S. L. & Madara, J. L. Partitioning of paracellular conductance along the ileal crypt–villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J. Membr. Biol. 80, 59–70 (1984).
Fihn, B. M., Sjoqvist, A. & Jodal, M. Permeability of the rat small intestinal epithelium along the villus–crypt axis: effects of glucose transport. Gastroenterology 119, 1029–1036 (2000).
Mora-Galindo, J. Maturation of tight junctions in guinea-pig cecal epithelium. Cell Tissue Res. 246, 169–175 (1986).
Suenaert, P. et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am. J. Gastroenterol. 97, 2000–2004 (2002).
Baert, F. J. et al. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology 116, 22–28 (1999).
Zolotarevsky, Y. et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123, 163–172 (2002).
Wang, F. et al. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 166, 409–419 (2005).
Clayburgh, D. R., Musch, M. W., Leitges, M., Fu, Y. X. & Turner, J. R. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J. Clin. Invest. 116, 2682–2694 (2006).
Wang, F. et al. IFN-γ-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 131, 1153–1163 (2006).
Clayburgh, D. R. et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Invest. 115, 2702–2715 (2005).
Graham, W. V. et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J. Biol. Chem. 281, 26205–26215 (2006).
Su, L. et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136, 551–563 (2009).
Marchiando, A. M. et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 189, 111–126 (2010).
Buschmann, M. M. et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell 24, 3056–3068 (2013).
Van Itallie, C. M., Fanning, A. S., Holmes, J. & Anderson, J. M. Occludin is required for cytokine-induced regulation of tight junction barriers. J. Cell Sci. 123, 2844–2852 (2010).
Westphal, J. K. et al. Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell. Mol. Life Sci. 67, 2057–2068 (2010).
Krug, S. M. et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 20, 3713–3724 (2009).
Ikenouchi, J., Sasaki, H., Tsukita, S., Furuse, M. & Tsukita, S. Loss of occludin affects tricellular localization of tricellulin. Mol. Biol. Cell 19, 4687–4693 (2008).
Kojima, T. et al. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J. Cell. Physiol. 225, 720–733 (2010).
Nayak, G. et al. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J. Clin. Invest. 123, 4036–4049 (2013).
Riazuddin, S. et al. Tricellulin is a tight-junction protein necessary for hearing. Am. J. Hum. Genet. 79, 1040–1051 (2006).
Chishti, M. S. et al. Splice-site mutations in the TRIC gene underlie autosomal recessive nonsyndromic hearing impairment in Pakistani families. J. Hum. Genet. 53, 101–105 (2008).
Kitajiri, S. I. et al. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biol. Open 3, 759–766 (2014).
Saitou, M. et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131–4142 (2000).
Schulzke, J. D. et al. Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta 1669, 34–42 (2005).
Olson, T. S. et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J. Exp. Med. 203, 541–552 (2006).
Maes, M. & Leunis, J. C. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol. Lett. 29, 902–910 (2008).
Quigley, E. M. Leaky gut — concept or clinical entity? Curr. Opin. Gastroenterol. 32, 74–79 (2016).
Odenwald, M. A. & Turner, J. R. Intestinal permeability defects: is it time to treat? Clin. Gastroenterol. Hepatol. 11, 1075–1083 (2013).
In, J. et al. Enterohemorrhagic Escherichia coli reduces mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell. Mol. Gastroenterol. Hepatol. 2, 48–62.e3 (2016).
Yuhan, R., Koutsouris, A., Savkovic, S. D. & Hecht, G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113, 1873–1882 (1997).
Sonoda, N. et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 147, 195–204 (1999).
Saitoh, Y. et al. Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 347, 775–778 (2015).
Hecht, G., Koutsouris, A., Pothoulakis, C., LaMont, J. T. & Madara, J. L. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology 102, 416–423 (1992).
Just, I. et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500–503 (1995).
Hollander, D. Crohn's disease — a permeability disorder of the tight junction? Gut 29, 1621–1624 (1988).
Pearson, A. D., Eastham, E. J., Laker, M. F., Craft, A. W. & Nelson, R. Intestinal permeability in children with Crohn's disease and coeliac disease. Br. Med. J. (Clin. Res. Ed.) 285, 20–21 (1982).
Ukabam, S. O., Clamp, J. R. & Cooper, B. T. Abnormal small intestinal permeability to sugars in patients with Crohn's disease of the terminal ileum and colon. Digestion 27, 70–74 (1983).
Schmitz, H. et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 116, 301–309 (1999).
Weber, C. R., Nalle, S. C., Tretiakova, M., Rubin, D. T. & Turner, J. R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 88, 1110–1120 (2008).
Blair, S. A., Kane, S. V., Clayburgh, D. R. & Turner, J. R. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab. Invest. 86, 191–201 (2006).
Wyatt, J., Vogelsang, H., Hubl, W., Waldhoer, T. & Lochs, H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341, 1437–1439 (1993).
D'Inca, R. et al. Intestinal permeability test as a predictor of clinical course in Crohn's disease. Am. J. Gastroenterol. 94, 2956–2960 (1999).
Tibble, J. A., Sigthorsson, G., Bridger, S., Fagerhol, M. K. & Bjarnason, I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 119, 15–22 (2000).
Kiesslich, R. et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 61, 1146–1153 (2012).
Madara, J. L. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J. Membr. Biol. 116, 177–184 (1990).
Hollander, D. et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 105, 883–885 (1986).
Jacobs, J. P. et al. A disease-associated microbial and metabolomics state in relatives of pediatric inflammatory bowel disease patients. Cell. Mol. Gastroenterol. Hepatol. 2, 750–766 (2016).
Li, X. et al. Microgeographic proteomic networks of the human colonic mucosa and their association with inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2, 567–583 (2016).
Buhner, S. et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut 55, 342–347 (2006).
Bjarnason, I., MacPherson, A. & Hollander, D. Intestinal permeability: an overview. Gastroenterology 108, 1566–1581 (1995).
Irvine, E. J. & Marshall, J. K. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology 119, 1740–1744 (2000).
Vetrano, S. et al. Unique role of junctional adhesion molecule-A in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 135, 173–184 (2008).
Turner, J. R. in Robbins and Cotran Pathologic Basis of Disease (eds Kumar, V., Abbas, A. K. & Aster, J. C.) 749–819 (Elsevier, 2014).
Hamilton, I., Cobden, I., Rothwell, J. & Axon, A. T. Intestinal permeability in coeliac disease: the response to gluten withdrawal and single-dose gluten challenge. Gut 23, 202–210 (1982).
Smecuol, E. et al. Gastrointestinal permeability in celiac disease. Gastroenterology 112, 1129–1136 (1997).
van Elburg, R. M., Uil, J. J., Mulder, C. J. & Heymans, H. S. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 34, 354–357 (1993).
Cummins, A. G. et al. Improvement in intestinal permeability precedes morphometric recovery of the small intestine in coeliac disease. Clin. Sci. (Lond.) 100, 379–386 (2001).
Vazquez-Roque, M. I. et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144, 903–911.e3 (2013).
Hall, E. J. & Batt, R. M. Abnormal permeability precedes the development of a gluten sensitive enteropathy in Irish setter dogs. Gut 32, 749–753 (1991).
Verdu, E. F. et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G217–G225 (2008).
Natividad, J. M. et al. Host responses to intestinal microbial antigens in gluten-sensitive mice. PLoS ONE 4, e6472 (2009).
Clemente, M. G. et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 52, 218–223 (2003).
Sander, G. R., Cummins, A. G., Henshall, T. & Powell, B. C. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 579, 4851–4855 (2005).
Fasano, A. et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355, 1518–1519 (2000).
Gopalakrishnan, S. et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides 35, 86–94 (2012).
Kelly, C. P. et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment. Pharmacol. Ther. 37, 252–262 (2013).
Monsuur, A. J. et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat. Genet. 37, 1341–1344 (2005).
Van Belzen, M. J. et al. A major non-HLA locus in celiac disease maps to chromosome 19. Gastroenterology 125, 1032–1041 (2003).
Wirth, J. A., Jensen, K. A., Post, P. L., Bement, W. M. & Mooseker, M. S. Human myosin-IXb, an unconventional myosin with a chimerin-like rho/rac GTPase-activating protein domain in its tail. J. Cell Sci. 109, 653–661 (1996).
Post, P. L., Bokoch, G. M. & Mooseker, M. S. Human myosin-IXb is a mechanochemically active motor and a GAP for rho. J. Cell Sci. 111, 941–950 (1998).
Hunt, K. A. et al. Lack of association of MYO9B genetic variants with coeliac disease in a British cohort. Gut 55, 969–972 (2006).
Amundsen, S. S. et al. Association analysis of MYO9B gene polymorphisms with celiac disease in a Swedish/Norwegian cohort. Hum. Immunol. 67, 341–345 (2006).
Wolters, V. M. et al. Replication of genetic variation in the MYO9B gene in Crohn's disease. Hum. Immunol. 72, 592–597 (2011).
van Bodegraven, A. A. et al. Genetic variation in myosin IXB is associated with ulcerative colitis. Gastroenterology 131, 1768–1774 (2006).
Cooney, R. et al. Association between genetic variants in myosin IXB and Crohn's disease. Inflamm. Bowel Dis. 15, 1014–1021 (2009).
Chandhoke, S. K. & Mooseker, M. S. A role for myosin IXb, a motor-RhoGAP chimera, in epithelial wound healing and tight junction regulation. Mol. Biol. Cell 23, 2468–2480 (2012).
Hegan, P. S. et al. Mice lacking myosin IXb, an inflammatory bowel disease susceptibility gene, have impaired intestinal barrier function and superficial ulceration in the ileum. Cytoskeleton (Hoboken) 73, 163–179 (2016).
Schumann, M. et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut 61, 220–228 (2012).
Szakal, D. N. et al. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch. 456, 245–250 (2010).
Menzies, I. S. et al. Abnormal intestinal permeability to sugars in villous atrophy. Lancet 2, 1107–1109 (1979).
Keating, J. et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut 37, 623–629 (1995).
Hermiston, M. L. & Gordon, J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 270, 1203–1207 (1995).
Jankowski, J. A. et al. Alterations in classical cadherins associated with progression in ulcerative and Crohn's colitis. Lab. Invest. 78, 1155–1167 (1998).
Smalley-Freed, W. G. et al. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J. Clin. Invest. 120, 1824–1835 (2010).
Schneider, M. R. et al. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS ONE 5, e14325 (2010).
Barrett, J. C. et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat. Genet. 41, 1330–1334 (2009).
Moran, C. J. et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm. Bowel Dis. 19, 115–123 (2013).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009).
Doecke, J. D. et al. Genetic susceptibility in IBD: overlap between ulcerative colitis and Crohn's disease. Inflamm. Bowel Dis. 19, 240–245 (2013).
Madsen, K. L. Inflammatory bowel disease: lessons from the IL-10 gene-deficient mouse. Clin. Invest. Med. 24, 250–257 (2001).
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Matharu, K. S. et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology 137, 1380–1390.e3 (2009).
Madsen, K. L. et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 5, 262–270 (1999).
Madsen, K. L. et al. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology 118, 1094–1105 (2000).
Kostic, A. D., Xavier, R. J. & Gevers, D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 146, 1489–1499 (2014).
Berg, D. J. et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology 123, 1527–1542 (2002).
Arrieta, M. C., Madsen, K. L., Field, C. J. & Meddings, J. B. Increasing small intestinal permeability worsens colitis in the IL-10−/− mouse and prevents the induction of oral tolerance to ovalbumin. Inflamm. Bowel Dis. 21, 8–18 (2015).
Arrieta, M. C., Madsen, K., Doyle, J. & Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 58, 41–48 (2009).
Storb, R. et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings — beneficial effect of a protective environment. N. Engl. J. Med. 308, 302–307 (1983).
Brown, G. R. et al. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology 116, 593–601 (1999).
Cooke, K. R. et al. Tumor necrosis factor- α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J. Clin. Invest. 102, 1882–1891 (1998).
Nalle, S. C. et al. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Sci. Transl Med. 6, 243ra87 (2014).
Bosi, E. et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 49, 2824–2827 (2006).
Meddings, J. B., Jarand, J., Urbanski, S. J., Hardin, J. & Gall, D. G. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am. J. Physiol. 276, G951–G957 (1999).
Tennyson, C. A. & Friedman, G. Microecology, obesity, and probiotics. Curr. Opin. Endocrinol. Diabetes Obes. 15, 422–427 (2008).
Pound, L. D. et al. Cathelicidin antimicrobial peptide: a novel regulator of islet function, islet regeneration, and selected gut bacteria. Diabetes 64, 4135–4147 (2015).
Daft, J. G. & Lorenz, R. G. Role of the gastrointestinal ecosystem in the development of Type 1 diabetes. Pediatr. Diabetes 16, 407–418 (2015).
Wen, L. et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455, 1109–1113 (2008).
Pozzilli, P., Signore, A., Williams, A. J. & Beales, P. E. NOD mouse colonies around the world — recent facts and figures. Immunol. Today 14, 193–196 (1993).
Watts, T. et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc. Natl Acad. Sci. USA 102, 2916–2921 (2005).
Sapone, A. et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55, 1443–1449 (2006).
Leffler, D. A. et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 107, 1554–1562 (2012).
Avansino, J. R., Chen, D. C., Woolman, J. D., Hoagland, V. D. & Stelzner, M. Engraftment of mucosal stem cells into murine jejunum is dependent on optimal dose of cells. J. Surg. Res. 132, 74–79 (2006).
Tait, I. S., Evans, G. S., Flint, N. & Campbell, F. C. Colonic mucosal replacement by syngeneic small intestinal stem cell transplantation. Am. J. Surg. 167, 67–72 (1994).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Aihara, E. et al. Epithelial regeneration after gastric ulceration causes prolonged cell-type alterations. Cell. Mol. Gastroenterol. Hepatol. 2, 625–647 (2016).
Engevik, A. C. et al. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell. Mol. Gastroenterol. Hepatol. 2, 605–624 (2016).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
Schepers, A. G. et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337, 730–735 (2012).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Kieckhaefer, J. et al. The RNA polymerase III subunit Polr3b is required for the maintenance of small intestinal crypts in mice. Cell. Mol. Gastroenterol. Hepatol. 2, 783–795 (2016).
Watanabe, N. et al. Requirement of Gαq/Gα11 signaling in the preservation of mouse intestinal epithelial homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2, 767–782 (2016).
Yamaoka, T. et al. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc. Natl Acad. Sci. USA 105, 11772–11777 (2008).
Zhao, J. et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology 132, 1331–1343 (2007).
Pinto, D. & Clevers, H. Wnt, stem cells and cancer in the intestine. Biol. Cell 97, 185–196 (2005).
Sansom, O. J. et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 18, 1385–1390 (2004).
Powell, A. E. et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012).
Wong, V. W. et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat. Cell Biol. 14, 401–408 (2012).
Dube, P. E. et al. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J. Clin. Invest. 122, 2780–2792 (2012).
He, W. Q. et al. Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 301, H584–H591 (2011).
He, W. Q. et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology 135, 610–620 (2008).
Zhang, W. C. et al. Myosin light chain kinase is necessary for tonic airway smooth muscle contraction. J. Biol. Chem. 285, 5522–5531 (2010).
Schumann, M. et al. Defective tight junctions in refractory celiac disease. Ann. N. Y. Acad. Sci. 1258, 43–51 (2012).
Noth, R. et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol. 11, 109 (2011).
Becker, C., Watson, A. J. & Neurath, M. F. Complex roles of caspases in the pathogenesis of inflammatory bowel disease. Gastroenterology 144, 283–293 (2013).
Washington, K. & Jagasia, M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum. Pathol. 40, 909–917 (2009).
Andre, F. et al. Assessment of the lactulose–mannitol test in Crohn's disease. Gut 29, 511–515 (1988).
Peeters, M. et al. Increased permeability of macroscopically normal small bowel in Crohn's disease. Dig. Dis. Sci. 39, 2170–2176 (1994).
Sundqvist, T., Magnusson, K. E., Sjodahl, R., Stjernstrom, I. & Tagesson, C. Passage of molecules through the wall of the gastrointestinal tract. II. Application of low-molecular weight polyethyleneglycol and a deterministic mathematical model for determining intestinal permeability in man. Gut 21, 208–214 (1980).
Arnott, I. D., Kingstone, K. & Ghosh, S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand. J. Gastroenterol. 35, 1163–1169 (2000).
May, G. R., Sutherland, L. M. & Meddings, J. B. Lactulose/mannitol permeability is increased in relatives of patients with Crohn's disease. Gastroenterology 102, A934 (1992).
Smecuol, E. et al. Sugar tests detect celiac disease among first-degree relatives. Am. J. Gastroenterol. 94, 3547–3552 (1999).
Secondulfo, M. et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig. Liver Dis. 36, 35–45 (2004).
Poritz, L. S. et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 140, 12–19 (2007).
Yan, Y. et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE 4, e6073 (2009).
Johansson, J. E. & Ekman, T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig. Dis. Sci. 52, 2340–2345 (2007).
Lee, A. S. et al. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia 53, 741–748 (2010).
Acknowledgements
The authors acknowledge NIH grants F30DK103511 (M.A.O.), T32HD007009 (M.A.O.), R01DK61931 (J.R.T.) and R01DK68271 (J.R.T.).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Odenwald, M., Turner, J. The intestinal epithelial barrier: a therapeutic target?. Nat Rev Gastroenterol Hepatol 14, 9–21 (2017). https://doi.org/10.1038/nrgastro.2016.169
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.169
This article is cited by
-
Polymeric DNase-I nanozymes targeting neutrophil extracellular traps for the treatment of bowel inflammation
Nano Convergence (2024)
-
Zinc glycine chelate ameliorates DSS-induced intestinal barrier dysfunction via attenuating TLR4/NF-κB pathway in meat ducks
Journal of Animal Science and Biotechnology (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)
-
Advancements in understanding bacterial enteritis pathogenesis through organoids
Molecular Biology Reports (2024)
-
Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review
Internal and Emergency Medicine (2024)