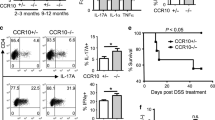
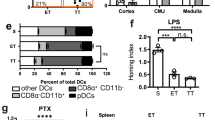
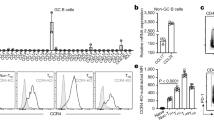
Key Points
-
Chemokines work together with adhesion molecules to coordinate the migration of IgA and IgG antibody-secreting cells (ASCs) to target effector tissues.
-
IgA ASCs that arise in the lymphoid tissues of the upper aerodigestive tract mainly traffic to non-intestinal mucosal sites, whereas IgA ASCs that develop in lymphoid tissues of the intestine can migrate to both intestinal and non-intestinal epithelial tissues. This process is mediated by differential expression of integrins and chemokine receptors.
-
Early ASCs downregulate the expression of chemokine receptors for lymphoid-tissue chemokines and upregulate the expression of chemokine receptors that are used for trafficking to effector sites.
-
Both IgG and IgA ASCs might use CXC-chemokine receptor 4 (CXCR4) for trafficking to the bone marrow.
-
IgG ASCs upregulate the expression of CXCR3, which they probably use for trafficking to sites of inflammation.
-
Upregulation of CC-chemokine receptor 9 (CCR9) expression by ASCs that are primed by small intestinal antigens probably focuses their migration back to the small intestine.
-
The expression of CCR10 is upregulated by IgA ASCs throughout the body and, together with specific integrins, allows IgA ASCs to disseminate to many effector tissues.
-
Further study will be required to assess the roles of chemokines in plasma-cell retention and survival, to elucidate the regulatory mechanisms that underlie the co-expression of IgA with mucosal chemokine receptors and IgG with inflammatory chemokine receptors, and to characterize the trafficking properties of IgE ASCs.
Abstract
Recent studies indicate that chemoattractant cytokines (chemokines), together with tissue-specific adhesion molecules, coordinate the migration of antibody-secreting cells (ASCs) from their sites of antigen-driven differentiation in lymphoid tissues to target effector tissues. Developing ASCs downregulate the expression of receptors for lymphoid tissue chemokines and selectively upregulate the expression of chemokine receptors that might target the migration of IgA ASCs to mucosal surfaces, IgG ASCs to sites of tissue inflammation and both types of ASC to the bone marrow — an important site for serum antibody production. By directing plasma-cell homing, chemokines might help to determine the character and efficiency of mucosal, inflammatory and systemic antibody responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brandtzaeg, P., Farstad, I. N. & Haraldsen, G. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol. Today 20, 267–277 (1999).
Calame, K. L. Plasma cells: finding new light at the end of B cell development. Nature Immunol. 2, 1103–1108 (2001).
Husband, A. J. & Gowans, J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J. Exp. Med. 148, 1146–1160 (1978).
McDermott, M. R. & Bienenstock, J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122, 1892–1898 (1979).
Rudzik, R., Clancy, R. L., Perey, D. Y., Day, R. P. & Bienenstock, J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J. Immunol. 114, 1599–1604 (1975).
Weisz-Carrington, P., Roux, M. E., McWilliams, M., Phillips-Quagliata J. M. & Lamm, M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J. Immunol. 123, 1705–1708 (1979).
Youngman, K. R. et al. Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J. Immunol. 168, 2173–2181 (2002). This paper shows that the intestinal homing receptor α 4 β 7 is tightly regulated during antigen-specific B-cell responses to infection of the small intestine with rotavirus and is expressed concurrently with the specific migration of antibody-secreting B cells (ASCs) to intestinal tissues.
Butcher, E. C., Williams, M., Youngman, K., Rott, L. & Briskin, M. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72, 209–253 (1999).
Farstad, I. N. et al. Human intestinal B-cell blasts and plasma cells express the mucosal homing receptor integrin α4β7 . Scand. J. Immunol. 42, 662–672 (1995).
Quiding-Jabrink, M. et al. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J. Clin. Invest. 99, 1281–1286 (1997). The authors describe differential expression of the intestinal homing receptor α 4 β 7 by circulating ASCs as a function of their isotype and site of induction in humans. Circulating antigen-specific IgA and IgG ASCs induced after oral, nasal or colonic immunization express α 4 β 7 , whereas IgG ASCs induced systemically largely lack α 4 β 7 expression. By contrast, expression of L-selectin is more efficiently induced after nasal or systemic immunization compared with oral or colonic immunization.
Medina, F., Segundo, C., Campos-Caro, A., Gonzalez-Garcia, I. & Brieva, J. A. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood 99, 2154–2161 (2002).
Finke, D., Baribaud, F., Diggelmann, H. & Acha-Orbea, H. Extrafollicular plasmablast B cells play a key role in carrying retroviral infection to peripheral organs. J. Immunol. 166, 6266–6275 (2001).
Rott, L. S., Briskin, M. J. & Butcher, E. C. Expression of α4β7 and E-selectin ligand by circulating memory B cells: implications for targeted trafficking to mucosal and systemic sites. J. Leukoc. Biol. 68, 807–814 (2000).
Czinn, S. J. & Lamm, M. E. Selective chemotaxis of subsets of B lymphocytes from gut-associated lymphoid tissue and its implications for the recruitment of mucosal plasma cells. J. Immunol. 136, 3607–3611 (1986).
Bowman, E. P. et al. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J. Exp. Med. 191, 1303–1318 (2000).
Warnock, R. A. et al. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 191, 77–88 (2000).
Stein, J. V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Cyster, J. G. et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176, 181–193 (2000).
Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416, 94–99 (2002).
Liao, F., Shirakawa, A. K., Foley, J. F., Rabin, R. L. & Farber, J. M. Human B cells become highly responsive to macrophage-inflammatory protein-3α/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J. Immunol. 168, 4871–4880 (2002).
Tanaka, Y. et al. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur. J. Immunol. 29, 633–642 (1999).
Bleul, C. C., Schultze, J. L. & Springer, T. A. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J. Exp. Med. 187, 753–762 (1998).
Krzysiek, R. et al. Regulation of CCR6 chemokine receptor expression and responsiveness to macrophage inflammatory protein-3α/CCL20 in human B cells. Blood 96, 2338–2345 (2000).
Roy, M. P., Kim, C. H. & Butcher, E. C. Cytokine control of memory B cell homing machinery. J. Immunol. 169, 1676–1682 (2002).
Forster, R., Wolf, I., Kaiser, E. & Lipp, M. Selective expression of the murine homologue of the G-protein-coupled receptor BLR1 in B cell differentiation, B cell neoplasia and defined areas of the cerebellum. Cell Mol Biol 40, 381–387 (1994).
Hargreaves, D. C. et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194, 45–56 (2001). This paper reports the downregulation of responsiveness to lymphoid-tissue chemokines during the development of ASCs, and an important role for CXC-chemokine receptor 4 (CXCR4) and CXC-chemokine ligand 12 (CXCL12) in plasma-cell localization to the bone marrow.
Hauser, A. E. et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 169, 1277–1282 (2002). This paper shows that IgG-secreting plasmablasts that are induced by systemic antigen upregulate responses to CXCR3 ligands, but lose chemokine responsiveness after a few days.
Kunkel, E. J. et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J. Clin. Invest . 111, 1001–1010 (2003). This paper reports on the expression of CC-chemokine receptor 10 (CCR10) — the receptor for the mucosal epithelial chemokine CCL28 — by IgA-secreting plasmablasts in the lymphoid tissues, blood and effector sites in humans and the concurrent downregulation of expression CCR6.
Egawa, T. et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity 15, 323–334 (2001).
Ma, Q. et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl Acad. Sci. USA 95, 9448–9453 (1998).
Lazarus, N. H. et al. A common mucosal chemokine (mucosa-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 170, 3799–3805 (2003). This paper shows the general responsiveness of IgA-secreting plasmablasts to CCL28, and the responsiveness of small intestinal ASCs to CCL25. They also show that terminally differentiated plasma cells retain chemokine-binding ability, but lose the ability to undergo chemotaxis.
Agace, W. W. et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr. Biol. 10, 325–328 (2000).
Nakayama, T. et al. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J. Immunol. 170, 1136–1140 (2003).
Berek, C. & Kim, H. J. B-cell activation and development within chronically inflamed synovium in rheumatoid and reactive arthritis. Semin. Immunol. 9, 261–268 (1997).
Weyand, C. M. & Goronzy, J. J. Ectopic germinal center formation in rheumatoid synovitis. Ann. NY Acad. Sci. 987, 140–149 (2003).
Berek, C. & Schroder, A. E. A germinal center-like reaction in the nonlymphoid tissue of the synovial membrane. Ann. NY Acad. Sci. 815, 211–217 (1997).
Farber, J. M. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol . 61, 246–257 (1997).
Bowman, E. P. et al. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J. Exp. Med. 195, 269–275 (2002).
Kunkel, E. J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Papadakis, K. A. et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165, 5069–5076 (2000).
Zabel, B. A. et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190, 1241–1256 (1999).
Pan, J. et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J. Immunol. 165, 2943–2949 (2000).
Wang, W. et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J. Biol. Chem. 275, 22313–22323 (2000).
Hieshima, K. et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 170, 1452–1461 (2003).
Kroese, F. G. et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1, 75–84 (1989).
Brandtzaeg, P., Baekkevold, E. S. & Morton, H. C. From B to A the mucosal way. Nature Immunol. 2, 1093–1094 (2001).
Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & Honjo, T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413, 639–643 (2001).
VanCott, J. L., Brim, T. A., Lunney, J. K. & Saif, L. J. Contribution of antibody-secreting cells induced in mucosal lymphoid tissues of pigs inoculated with respiratory or enteric strains of coronavirus to immunity against enteric coronavirus challenge. J. Immunol. 152, 3980–3990 (1994).
Czerkinsky, C., Svennerholm, A. M., Quiding, M., Jonsson, R. & Holmgren, J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect. Immun. 59, 996–1001 (1991).
Kurono, Y. et al. Nasal immunization induces Haemophilus influenzae-specific TH1 and TH2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J. Infect. Dis. 180, 122–132 (1999).
Rudin, A., Riise, G. C. & Holmgren, J. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect. Immun. 67, 2884–2890 (1999).
Johansson, E. L., Wassen, L., Holmgren, J., Jertborn, M. & Rudin, A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect. Immun. 69, 7481–7486 (2001).
Homey, B. et al. CCL27–CCR10 interactions regulate T cell-mediated skin inflammation. Nature Med. 8, 157–165 (2002).
Izadpanah, A., Dwinell, M. B., Eckmann, L., Varki, N. M. & Kagnoff, M. F. Regulated MIP-3α/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G710–G719 (2001).
Nakayama, T. et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3α/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int. Immunol. 13, 95–103 (2001).
Reiss, Y., Proudfoot, A. E., Power, C. A., Campbell, J. J. & Butcher, E. C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194, 1541–1547 (2001).
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Acknowledgements
We thank N.H. Lazarus for input into this manuscript. This work was supported by the United States Public Health Service and the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunkel, E., Butcher, E. Plasma-cell homing. Nat Rev Immunol 3, 822–829 (2003). https://doi.org/10.1038/nri1203
Issue Date:
DOI: https://doi.org/10.1038/nri1203
This article is cited by
-
Supplying the trip to antibody production—nutrients, signaling, and the programming of cellular metabolism in the mature B lineage
Cellular & Molecular Immunology (2022)
-
IgA glycosylation and immune complex formation in IgAN
Seminars in Immunopathology (2021)
-
Oral vitamin A supplementation of porcine epidemic diarrhea virus infected gilts enhances IgA and lactogenic immune protection of nursing piglets
Veterinary Research (2019)
-
BLT1 mediates commensal bacteria-dependent innate immune signals to enhance antigen-specific intestinal IgA responses
Mucosal Immunology (2019)
-
B cells and antibody production in melanoma
Mammalian Genome (2018)