Key Points
-
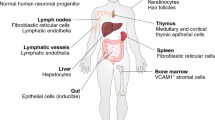
The receptor for the cytokine interleukin-21 (IL-21) contains the common cytokine-receptor γ-chain, so IL-21 is one of the cytokines that has its effects ablated in individuals with X-linked severe combined immunodeficiency. Although IL-21 is expressed only by CD4+ T cells, its receptor is found at the cell surface of all T cells, B cells, natural killer (NK) cells, dendritic cells (DCs) and keratinocytes, thereby implying that IL-21 has a diverse range of effects.
-
The effects of IL-21 on B cells are context dependent, and they include the induction of apoptosis in naive B cells and, after co-activation with signals from T cells, the induction of differentiation of B cells into plasma cells, through upregulation of expression of B-lymphocyte-induced maturation protein 1 (BLIMP1).
-
IL-21 augments the proliferation of NK cells and leads to the acquisition of a functional cytotoxic state. In vivo treatment with IL-21 leads to increased cytotoxic activity of NK cells and to the induction of a strong antitumour activity in several models of cancer.
-
IL-21 has a synergistic effect on the clonal expansion of CD8+ T cells when delivered in combination with either IL-7 or IL-15. This effect correlates with the ability of IL-21 to promote both increased cytotoxic activity of CD8+ T cells and potent antitumour effects by these cells.
-
IL-21 can negatively control immune responses through its inhibitory effects on DC differentiation and its apoptotic effects on activated NK cells.
-
Our knowledge of the basic biology of IL-21 indicates that there are clinical situations, such as those in autoimmune disease, allergy and cancer, in which either ablation or augmentation of IL-21-mediated signalling might have therapeutic benefit.
Abstract
The interleukin-21 (IL-21)–IL-21-receptor system was discovered in 2000. It was immediately of great interest because of the homology of IL-21 to IL-2, IL-4 and IL-15, and of the IL-21-receptor subunit IL-21R to the β-subunit of the IL-2 receptor, and because the IL-21 receptor also contains the common cytokine-receptor γ-chain, the protein that is mutated in X-linked severe combined immunodeficiency. As we discuss, IL-21 has pleiotropic actions, from augmenting the proliferation of T cells and driving the differentiation of B cells into memory cells and terminally differentiated plasma cells to augmenting the activity of natural killer cells. Moreover, it has antitumour activity and might have a role in the development of autoimmunity, so these findings have implications for the treatment of cancer and autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Leonard, W. J. in Fundamental Immunology 5th Edn (ed. Paul, W. E.) 701–747 (Lippincott Williams & Wilkins, Philadelphia, 2003)
Leonard, W. J. Cytokines and immunodeficiency diseases. Nature Rev. Immunol. 1, 200–208 (2001).
Noguchi, M. et al. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73, 147–157 (1993).
Russell, S. M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Ozaki, K., Kikly, K., Michalovich, D., Young, P. R. & Leonard, W. J. Cloning of a type I cytokine receptor most related to the IL-2 receptor β chain. Proc. Natl Acad. Sci. USA 97, 11439–11444 (2000). This was the first report to identify the IL-21 receptor as a novel type I cytokine receptor and to describe its structural features and signal-transduction pathways.
Parrish-Novak, J. et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408, 57–63 (2000). This paper identifies the IL-21 receptor and its ligand and presents the first analysis of the proliferative and functional effects of IL-21 on T cells, B cells and NK cells.
Asao, H. et al. The common γ-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167, 1–5 (2001).
Habib, T., Senadheera, S., Weinberg, K. & Kaushansky, K. The common γ chain (γc) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry 41, 8725–8731 (2002).
Bennett, F. et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 170, 711–718 (2003).
Strengell, M., Sareneva, T., Foster, D., Julkunen, I. & Matikainen, S. IL-21 up-regulates the expression of genes associated with innate immunity and TH1 response. J. Immunol. 169, 3600–3605 (2002).
Strengell, M. et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-γ production in human NK and T cells. J. Immunol. 170, 5464–5469 (2003).
Lin, J. -X. et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 2, 331–339 (1995).
Lin, J. -X., Mietz, J., Modi, W. S., John, S. & Leonard, W. J. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J. Biol. Chem. 271, 10738–10744 (1996).
Hou, J. et al. An interleukin-4-induced transcription factor: IL-4 Stat. Science 265, 1701–1706 (1994).
Quelle, F. W. et al. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol. Cell. Biol. 15, 3336–3343 (1995).
Nielsen, M., Svejgaard, A., Skov, S. & Odum, N. Interleukin-2 induces tyrosine phosphorylation and nuclear translocation of stat3 in human T lymphocytes. Eur. J. Immunol. 24, 3082–3086 (1994).
Chin, Y. E., Kitagawa, M., Kuida, K., Flavell, R. A. & Fu, X. Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cell. Biol. 17, 5328–5337 (1997).
Stephanou, A. & Latchman, D. S. STAT-1: a novel regulator of apoptosis. Int. J. Exp. Pathol. 84, 239–244 (2003).
Yu, C. L. et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269, 81–83 (1995).
Bromberg, J. F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999).
Bromberg, J. & Darnell, J. E. Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19, 2468–2473 (2000).
Jin, H., Carrio, R., Yu, A. & Malek, T. R. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 173, 657–665 (2004). This paper describes the context-dependent effects of IL-21 on the B-cell lineage and identifies a BIM-dependent, IL-21-mediated apoptotic pathway in T-cell-independent B-cell responses.
Brandt, K., Bulfone-Paus, S., Foster, D. C. & Ruckert, R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood 102, 4090–4098 (2003).
Brandt, K. et al. Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J. Invest. Dermatol. 121, 1379–1382 (2003).
Distler, J. H. et al. Expression of interleukin-21 receptor in epidermis from patients with systemic sclerosis. Arthritis Rheum. 52, 856–864 (2005).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002). This paper describes the defective antibody production in Il21r−/− mice and shows that IL-21 and IL-4 cooperate in the regulation of antibody production.
Eberl, M., Engel, R., Beck, E. & Jomaa, H. Differentiation of human γδ T cells towards distinct memory phenotypes. Cell. Immunol. 218, 1–6 (2002).
Strengell, M., Julkunen, I. & Matikainen, S. IFN-α regulates IL-21 and IL-21R expression in human NK and T cells. J. Leukoc. Biol. 76, 416–422 (2004).
Zeng, R. et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 201, 139–148 (2005). This report describes the cooperative effect of IL-21 and IL-15 on CD8+ T-cell proliferation and antitumour activity towards established melanomas.
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-TH1/TH2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Wurster, A. L. et al. Interleukin 21 is a T helper (TH) cell 2 cytokine that specifically inhibits the differentiation of naive TH cells into interferon γ-producing TH1 cells. J. Exp. Med. 196, 969–977 (2002).
Kim, H. -P., Korn, L. L., Gamero, A. M. & Leonard, W. J. Calcium-dependent activation of interleukin-21 gene expression in T cells. J. Biol. Chem. 280, 25291–25297 (2005).
Mehta, D. S., Wurster, A. L., Weinmann, A. S. & Grusby, M. J. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc. Natl Acad. Sci. USA 102, 2016–2021 (2005).
Kasaian, M. T. et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 16, 559–569 (2002).
Kuhn, R., Rajewsky, K. & Muller, W. Generation and analysis of interleukin-4 deficient mice. Science 254, 707–710 (1991).
Suto, A. et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line Cε transcription of IL-4-stimulated B cells. Blood 100, 4565–4573 (2002).
Oettgen, H. C. Regulation of the IgE isotype switch: new insights on cytokine signals and the functions of ε germline transcripts. Curr. Opin. Immunol. 12, 618–623 (2000).
Kaplan, M. H., Schindler, U., Smiley, S. T. & Grusby, M. J. Stat6 is required for mediating responses to IL-4 and for development of TH2 cells. Immunity 4, 313–319 (1996).
Cao, X. et al. Characterization of cDNAs encoding the murine interleukin 2 receptor (IL-2R) γ chain: chromosomal mapping and tissue specificity of IL-2R γ chain expression. Proc. Natl Acad. Sci. USA 90, 8464–8468 (1993).
DiSanto, J. P., Rieux-Laucat, F., Dautry-Varsat, A., Fischer, A. & de Saint Basile, G. Defective human interleukin 2 receptor γ chain in an atypical X chromosome-linked severe combined immunodeficiency with peripheral T cells. Proc. Natl Acad. Sci. USA 91, 9466–9470 (1994).
Puel, A., Ziegler, S. F., Buckley, R. H. & Leonard, W. J. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nature Genet. 20, 394–397 (1998).
Waldmann, T. A. The multi-subunit interleukin-2 receptor. Annu. Rev. Biochem. 58, 875–911 (1989).
Conley, M. E. et al. Nonrandom X chromosome inactivation in B cells from carriers of X chromosome-linked severe combined immunodeficiency. Proc. Natl Acad. Sci. USA 85, 3090–3094 (1988).
Akashi, K., Kondo, M., von Freeden-Jeffry, U., Murray, R. & Weissman, I. L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89, 1033–1041 (1997).
Otani, H., Erdos, M. & Leonard, W. J. Tyrosine kinase(s) regulate apoptosis and bcl-2 expression in a growth factor-dependent cell line. J. Biol. Chem. 268, 22733–22736 (1993).
Mehta, D. S. et al. IL-21 induces the apoptosis of resting and activated primary B cells. J. Immunol. 170, 4111–4118 (2003).
Ozaki, K. et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173, 5361–5371 (2004). This report shows that IL-21 initiates the development of B cells into plasma cells through directly inducing expression of the master regulator of this process, BLIMP1.
Lenardo, M. et al. Mature T lymphocyte apoptosis — immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17, 221–253 (1999).
Refaeli, Y., Van Parijs, L., London, C. A., Tschopp, J. & Abbas, A. K. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8, 615–623 (1998).
Pene, J. et al. IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 172, 5154–5157 (2004).
Calame, K. L., Lin, K. I. & Tunyaplin, C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 21, 205–230 (2003).
Shaffer, A. L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Murphy, E. D. & Roths, J. B. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 22, 1188–1194 (1979).
Cao, X. et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity 2, 223–238 (1995).
DiSanto, J. P., Muller, W., Guy-Grand, D., Fischer, A. & Rajewsky, K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor γ chain. Proc. Natl Acad. Sci. USA 92, 377–381 (1995).
Suzuki, H. et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268, 1472–1476 (1995).
Lodolce, J. P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Kennedy, M. K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Sivori, S. et al. IL-21 induces both rapid maturation of human CD34+ cell precursors towards NK cells and acquisition of surface killer Ig-like receptors. Eur. J. Immunol. 33, 3439–3447 (2003).
Sivori, S. et al. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc. Natl Acad. Sci. USA 99, 4526–4531 (2002).
Vosshenrich, C. A. et al. Roles for common cytokine receptor γ-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 174, 1213–1221 (2005).
Toomey, J. A., Gays, F., Foster, D. & Brooks, C. G. Cytokine requirements for the growth and development of mouse NK cells in vitro. J. Leukoc. Biol. 74, 233–242 (2003).
Brady, J., Hayakawa, Y., Smyth, M. J. & Nutt, S. L. IL-21 induces the functional maturation of murine NK cells. J. Immunol. 172, 2048–2058 (2004).
Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature Immunol. 1, 426–432 (2000).
Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Andrade, F. et al. Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity 8, 451–460 (1998).
Wang, G. et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 63, 9016–9022 (2003).
Pelletier, M., Bouchard, A. & Girard, D. In vivo and in vitro roles of IL-21 in inflammation. J. Immunol. 173, 7521–7530 (2004).
Anderson, M. S. & Bluestone, J. A. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23, 447–485 (2005).
Denny, P. et al. Mapping of the IDDM locus Idd3 to a 0.35-cM interval containing the interleukin-2 gene. Diabetes 46, 695–700 (1997).
King, C., Ilic, A., Koelsch, K. & Sarvetnick, N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117, 265–277 (2004).
Vollmer, T. L. et al. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J. Immunol. 174, 2696–2701 (2005).
Overwijk, W. W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003).
Klebanoff, C. A. et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl Acad. Sci. USA 101, 1969–1974 (2004).
Ugai, S. et al. Expression of the interleukin-21 gene in murine colon carcinoma cells generates systemic immunity in the inoculated hosts. Cancer Gene Ther. 10, 187–192 (2003).
Ugai, S. et al. Transduction of the IL-21 and IL-23 genes in human pancreatic carcinoma cells produces natural killer cell-dependent and -independent antitumor effects. Cancer Gene Ther. 10, 771–778 (2003).
Ma, H. L. et al. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-γ. J. Immunol. 171, 608–615 (2003).
Di Carlo, E. et al. IL-21 induces tumor rejection by specific CTL and IFN-γ-dependent CXC chemokines in syngeneic mice. J. Immunol. 172, 1540–1547 (2004).
Moroz, A. et al. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J. Immunol. 173, 900–909 (2004). This paper describes the unique ability of IL-21 to induce long-term immunity to tumours through its anti-apoptotic and survival effects on CD8+ T cells.
Shrikant, P. & Mescher, M. F. Opposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J. Immunol. 169, 1753–1759 (2002).
Kishida, T. et al. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol. Ther. 8, 552–558 (2003).
Schorle, H. et al. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352, 621–624 (1991).
Kundig, T. M. et al. Immune responses in interleukin-2 deficient mice. Science 262, 1059–1061 (1993).
Sadlack, B., et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75, 253–261 (1993).
Kopf, M., et al. Disruption of the murine IL-4 gene blocks TH2 cytokine responses. Nature 362, 245–248 (1993).
von Freeden-Jeffry, U. et al. Lymphopenia in interleukin-7 deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181, 1519–1526 (1995).
Townsend, J. M. et al. IL-9 deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13, 573–583 (2000).
McKenzie, G. J. et al. Impaired development of TH2 cells in IL-13 deficient mice. Immunity 9, 423–432 (1998).
Willerford, D. M. et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3, 521–530 (1995).
Roifman, C. M. Human IL-2 receptor α chain deficiency. Pediatr. Res. 48, 6–11 (2000).
Noben-Trauth, N. et al. An IL-4 independent pathway for CD4+ T cell IL-4 production revealed in IL-4 receptor deficient mice. Proc. Natl Acad. Sci. USA 94, 10838–10843 (1997).
Peschon, J. et al. Early lymphocyte expansion is severely impaired in interleukin-7 receptor deficient mice. J. Exp. Med. 180, 1955–1960 (1994).
Acknowledgements
The authors thank J.-X. Lin, H. C. Morse, P. Lipsky, R. Ettinger and A. Al Shami for crucial discussions and/or comments. This research was supported by the Intramural Research Programs of the National Heart, Lung, and Blood Institute, National Institutes of Health (United States).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- TYPE I CYTOKINE
-
A member of a family of cytokines that all have a four αhelical bundle structure and signal through type-I-cytokine receptors by mechanisms that include the Janus activated kinase (JAK)–signal transducer and activator of transcription (STAT) pathway. These cytokines include growth hormone, prolactin, thrombopoietin, erythropoietin and many interleukins.
- COMMON CYTOKINE-RECEPTOR γ-CHAIN
-
(γc). A chain that is present in type-I-cytokine receptors. It was first discovered as the γ-chain of the interleukin-2 (IL-2) receptor and was subsequently shown also to be present in the receptors for IL-4, IL-7, IL-9, IL-15 and IL-21. It is mutated in humans with X-linked severe combined immunodeficiency.
- EXPRESSED-SEQUENCE-TAG-BASED SEQUENCING
-
An expressed sequence tag (EST) is a partial cDNA sequence that can provide information to identify and isolate a complete gene.
- T-CELL-DEPENDENT ANTIGEN
-
A protein antigen that needs to be recognized by T helper cells (in the context of MHC molecules) and requires cooperation between these antigen-specific T cells and B cells for a specific antibody response to be generated.
- CLASS SWITCHING
-
A region-specific recombination process that occurs in antigen-activated B cells. This occurs between switch-region DNA sequences and results in a change in the class of antibody that is produced — from IgM to either IgG, IgA or IgE. This imparts flexibility to the humoral immune response and allows it to exploit the different capacities of these antibody classes to activate the appropriate downstream effector mechanisms.
- X-CHROMOSOME INACTIVATION
-
A process in which one of the two X chromosomes in females undergoes heterochromatization in diploid cells, resulting in its 'inactivation'. This process can have important genetic consequences in individuals who have a defective gene on one of their two X chromosomes.
- ACTIVATION-INDUCED CELL DEATH
-
(AICD). A process in which activated T cells undergo cell death after engagement of death receptors, such as CD95 or the tumour-necrosis-factor receptor, or after exposure to reactive oxygen species.
- ANNEXIN V
-
A molecule that binds phosphatidylserine, which is usually located on the inner leaflet of the plasma membrane but flips to the outer leaflet during apoptosis. Positive staining with annexin V is an indicator of apoptosis.
- POST-SWITCH B CELL
-
A B cell that no longer expresses IgD or IgM but, instead, expresses IgG at the cell surface.
- BCL-1 B-CELL LINE
-
A cell line that was established from a spontaneous mouse B-cell lymphoma (BCL). These cells can be induced to undergo differentiation into plasma cells in vitro.
- SYSTEMIC LUPUS ERYTHEMATOSUS
-
(SLE). An autoimmune disease in which autoantibodies that are specific for DNA, RNA or proteins associated with nucleic acids form immune complexes that damage small blood vessels, especially in the kidney. Patients with SLE generally have abnormal B- and T-cell function.
- EXPERIMENTAL ALLERGIC ENCEPHALOMYELITIS
-
(EAE). An experimental model for the human disease multiple sclerosis. Autoimmune disease is induced in experimental animals by immunization with myelin or peptides derived from myelin. The animals develop a paralytic disease with inflammation and demyelination in the brain and spinal cord.
Rights and permissions
About this article
Cite this article
Leonard, W., Spolski, R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol 5, 688–698 (2005). https://doi.org/10.1038/nri1688
Issue Date:
DOI: https://doi.org/10.1038/nri1688
This article is cited by
-
Anti-aquaporin-4 immune complex stimulates complement-dependent Th17 cytokine release in neuromyelitis optica spectrum disorders
Scientific Reports (2024)
-
The role of Th17 cells in the pathogenesis and treatment of breast cancer
Cancer Cell International (2022)
-
Cytokine-enhanced cytolytic activity of exosomes from NK Cells
Cancer Gene Therapy (2022)
-
Functional implications of the CpG island methylation in the pathogenesis of celiac disease
Molecular Biology Reports (2022)
-
Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy
Cellular Oncology (2022)