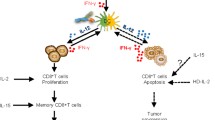
Key Points
-
Interleukin-7 (IL-7) is required for T cell development in mice and humans and is produced by stromal tissues rather than activated lymphocytes. Under normal conditions, IL-7 is a limiting resource for T cells, but it accumulates during lymphopenic conditions. IL-7 signals through a heterodimeric receptor consisting of the IL-7 receptor α-chain (IL-7Rα) and the common cytokine receptor γ-chain (γc).
-
IL-7 is not required for human B cell development in fetal life, but it affects early B cell progenitors and contributes to B cell development under normal conditions.
-
IL-7 has also been recently demonstrated to regulate lymphoid tissue inducer (LTi) cells, which induce the development of secondary lymphoid organs and can induce tertiary lymphoid tissue postnatally in settings of chronic inflammation.
-
In animals, IL-7 therapy enhances the effectiveness of adoptive immunotherapy for cancer, enhances vaccine responses and enhances viral clearance in the setting of acute and chronic infections.
-
In mature T cells, IL-7Rα is most highly expressed on recent thymic emigrants, maintained on naive T cells, downregulated upon T cell activation, and re-expressed on memory T cell subsets. As a result, treatment with recombinant human IL-7 (rhIL-7) preferentially expands recent thymic emigrants and naive T cells, as well as central memory T cells, but largely spares senescent T cells and regulatory T cells. This results in increased repertoire diversity following rhIL-7 therapy in humans.
-
Clinical results with rhIL-7 thus far have shown it to be well tolerated with dose-dependent increases in T cell numbers that persist long after the cytokine is cleared. Based on the pharmacological and biological properties demonstrated thus far, IL-7 is particularly well-suited as a therapy for conditions associated with lymphocyte immunodeficiency.
-
Multiple trials are ongoing or planned in HIV infection, other chronic infections (including hepatitis B and C), cancer (including as an adjuvant to immune-based therapies), post-haematopoietic stem cell transplantation and ageing.
Abstract
Interleukin-7 (IL-7) is required for T cell development and for maintaining and restoring homeostasis of mature T cells. IL-7 is a limiting resource under normal conditions, but it accumulates during lymphopaenia, leading to increased T cell proliferation. The administration of recombinant human IL-7 to normal or lymphopenic mice, non-human primates and humans results in widespread T cell proliferation, increased T cell numbers, modulation of peripheral T cell subsets and increased T cell receptor repertoire diversity. These effects raise the prospect that IL-7 could mediate therapeutic benefits in several clinical settings. This Review summarizes the biology of IL-7 and the results of its clinical use that are available so far to provide a perspective on the opportunities for clinical application of this cytokine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Guimond, M. et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nature Immunol. 10, 149–157 (2009). The first definitive evidence that the increased levels of IL-7 during lymphopaenia are the result of decreased consumption rather than increased production. This study also identifies IL-7-mediated signalling on DCs as a modulator of T cell homeostasis.
Pellegrini, M. et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nature Med. 15, 528–536 (2009). This study provides mechanistic insight into the vaccine adjuvant effect of IL-7 and increases the known targets of IL-7-mediated signalling to include negative regulators of the T cell response such as CBL-B and SMURF2.
Park, J. H. et al. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21, 289–302 (2004). This study established IL-7 as a limiting resource for T cells.
Fry, T. J. et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 101, 2294–2299 (2003). The first demonstration that the effects of in vivo IL-7 administration extend to non-human primates. This study also showed that IL-7-mediated signalling downregulates expression of IL-7Rα.
Khaled, A. R. & Durum, S. K. Death and Baxes: mechanisms of lymphotrophic cytokines. Immunol. Rev. 193, 48–57 (2003).
Jiang, Q. et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16, 513–533 (2005).
Puel, A., Ziegler, S. F., Buckley, R. H. & Leonard, W. J. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nature Genet. 20, 394–397 (1998). The first description in humans of SCID due to deficiency of IL-7Rα signalling. This study identified important differences in lymphocyte development between mice and humans.
Cunningham-Rundles, C. & Ponda, P. P. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nature Rev. Immunol. 5, 880–892 (2005).
Mazzucchelli, R. & Durum, S. K. Interleukin-7 receptor expression: intelligent design. Nature Rev. Immunol. 7, 144–154 (2007). A definitive review of the role of IL-7Rα in T cell development.
Akashi, K., Kondo, M., von Freeden-Jeffry, U., Murray, R. & Weissman, I. L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89, 1033–1041 (1997).
Maraskovsky, E. et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89, 1011–1019 (1997).
Pellegrini, M. et al. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J. Exp. Med. 200, 1189–1195 (2004).
Khaled, A. R. et al. Bax deficiency partially corrects interleukin-7 receptor-α deficiency. Immunity 17, 561–573 (2002).
Al-Shami, A. et al. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med. 200, 159–168 (2004).
Vang, K. B. et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 181, 3285–3290 (2008).
Mazzucchelli, R. et al. Development of regulatory T cells requires IL-7Rα stimulation by IL-7 or TSLP. Blood 112, 3283–3292 (2008).
Bayer, A. L., Lee, J. Y., de la Barrera, A., Surh, C. D. & Malek, T. R. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J. Immunol. 181, 225–234 (2008).
Herzog, S., Reth, M. & Jumaa, H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nature Rev. Immunol. 9, 195–205 (2009).
Parrish, Y. K. et al. IL-7 dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J. Immunol. 182, 4255–4266 (2009).
Shriner, A. K., Liu, H., Sun, G., Guimond, M. & Alugupalli, K. R. IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J. Immunol. 185, 525–531 (2010).
Kikuchi, K., Lai, A. Y., Hsu, C. L. & Kondo, M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J. Exp. Med. 201, 1197–1203 (2005).
Johnson, K. et al. Regulation of immunoglobulin light-chain recombination by the transcription factor IRF-4 and the attenuation of interleukin-7 signaling. Immunity 28, 335–345 (2008).
Corcoran, A. E., Riddell, A., Krooshoop, D. & Venkitaraman, A. R. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature 391, 904–907 (1998).
Bertolino, E. et al. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nature Immunol. 6, 836–843 (2005).
Malin, S. et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nature Immunol. 11, 171–179 (2010).
Brown, V. I. et al. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc. Natl Acad. Sci. USA 100, 15113–15118 (2003).
Yoda, A. et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc. Natl Acad. Sci. USA 107, 252–257 (2010).
Liu, Y. J. et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 25, 193–219 (2007).
Vogt, T. K., Link, A., Perrin, J., Finke, D. & Luther, S. A. Novel function for interleukin-7 in dendritic cell development. Blood 113, 3961–3968 (2009).
Vosshenrich, C. A. et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nature Immunol. 7, 1217–1224 (2006).
Ribeiro, V. S. et al. Cutting edge: Thymic NK cells develop independently from T cell precursors. J. Immunol. 185, 4993–4997 (2010).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nature Immunol. 5, 64–73 (2004).
Luther, S. A., Ansel, K. M. & Cyster, J. G. Overlapping roles of CXCL13, interleukin 7 receptor-α, and CCR7 ligands in lymph node development. J. Exp. Med. 197, 1191–1198 (2003).
Nishikawa, S., Honda, K., Vieira, P. & Yoshida, H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 195, 72–80 (2003).
Meier, D. et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26, 643–654 (2007). This study identifies the importance of IL-7 for the homeostasis of LTi cells and, therefore, the ability of IL-7 to regulate SLO development.
Schmutz, S. et al. Cutting edge: IL-7 regulates the peripheral pool of adult RORγ+ lymphoid tissue inducer cells. J. Immunol. 183, 2217–2221 (2009).
Tsuji, M. et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Moyron-Quiroz, J. E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nature Med. 10, 927–934 (2004).
Aloisi, F. & Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nature Rev. Immunol. 6, 205–217 (2006).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Cupedo, T. et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+CD127+ natural killer-like cells. Nature Immunol. 10, 66–74 (2009).
Vonarbourg, C. et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33, 736–751 (2010).
Cella, M., Otero, K. & Colonna, M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity. Proc. Natl Acad. Sci. USA 107, 10961–10966 (2010).
Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nature Immunol. 1, 426–432 (2000). The first demonstration that IL-7 is required for the homeostatic proliferation of CD8+ T cells during lymphopenic conditions.
Takada, K. & Jameson, S. C. Naive T cell homeostasis: from awareness of space to a sense of place. Nature Rev. Immunol. 9, 823–832 (2009).
Ouyang, W., Beckett, O., Flavell, R. A. & Li, M. O. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity 30, 358–371 (2009).
Grenningloh, R. et al. Ets-1 maintains IL-7 receptor expression in peripheral T cells. J. Immunol. 186, 969–976 (2010).
Matsue, H., Bergstresser, P. R. & Takashima, A. Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J. Immunol. 151, 6012–6019 (1993).
Thang, P. H. et al. The role of IL-1β in reduced IL-7 production by stromal and epithelial cells: a model for impaired T-cell numbers in the gut during HIV-1 infection. J. Intern. Med. 268, 181–193 (2010).
Watanabe, M. et al. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest. 95, 2945–2953 (1995).
Sawa, Y. et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity 30, 447–457 (2009).
Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 4, 1191–1198 (2003).
Tan, J. T. et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195, 1523–1532 (2002).
Kieper, W. C. et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195, 1533–1539 (2002).
Dardalhon, V. et al. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl Acad. Sci. USA 98, 9277–9282 (2001).
Swainson, L. et al. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood 109, 1034–1042 (2007).
Ernst, B., Lee, D. S., Chang, J. M., Sprent, J. & Surh, C. D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11, 173–181 (1999).
Goldrath, A. W. & Bevan, M. J. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11, 183–190 (1999).
Paiardini, M. et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174, 2900–2909 (2005).
Seddiki, N. et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 (2006).
Liu, W. et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ TReg cells. J. Exp. Med. 203, 1701–1711 (2006).
Simonetta, F. et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur. J. Immunol. 40, 2528–2538 (2010).
Bolotin, E., Annett, G., Parkman, R. & Weinberg, K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 23, 783–788 (1999). The first observation that serum IL-7 levels are increased during lymphopaenia after bone marrow transplantation. These findings were identified as a general feature of lymphopaenia with the discovery of increased IL-7 levels in other clinical conditions associated with T cell deficiency in references 65 and 66.
Fry, T. J. et al. A potential role for interleukin-7 in T-cell homeostasis. Blood 97, 2983–2990 (2001).
Napolitano, L. A. et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nature Med. 7, 73–79 (2001).
Spivak, J. L. Erythropoietin: from bench to bedside. Trans. Am. Clin. Climatol. Assoc. 102, 232–242 (1991).
Kuter, D. J. & Begley, C. G. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood 100, 3457–3469 (2002).
Takatani, H. et al. Levels of recombinant human granulocyte colony-stimulating factor in serum are inversely correlated with circulating neutrophil counts. Antimicrob. Agents Chemother. 40, 988–991 (1996).
Hakim, F. T. et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J. Clin. Invest. 115, 930–939 (2005).
Mackall, C. L. et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 89, 3700–3707 (1997).
Komschlies, K. L., Grzegorzewski, K. J. & Wiltrout, R. H. Diverse immunological and hematological effects of interleukin 7: implications for clinical application. J. Leukoc. Biol. 58, 623–633 (1995).
Storek, J. et al. Interleukin-7 improves CD4 T-cell reconstitution after autologous CD34 cell transplantation in monkeys. Blood 101, 4209–4218 (2003).
Fry, T. J., Christensen, B. L., Komschlies, K. L., Gress, R. E. & Mackall, C. L. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood 97, 1525–1533 (2001).
Mackall, C. L. et al. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood 97, 1491–1497 (2001).
Morrissey, P. J. et al. Administration of IL-7 to mice with cyclophosphamide-induced lymphopenia accelerates lymphocyte repopulation. J. Immunol. 146, 1547–1552 (1991).
Bolotin, E., Smogorzewska, M., Smith, S., Widmer, M. & Weinberg, K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood 88, 1887–1894 (1996).
Andrew, D. & Aspinall, R. IL-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J. Immunol. 166, 1524–1530 (2001).
Okamoto, Y., Douek, D. C., McFarland, R. D. & Koup, R. A. Effects of exogenous interleukin-7 on human thymus function. Blood 99, 2851–2858 (2002).
Min, D. et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood 99, 4592–4600 (2002).
Seggewiss, R. et al. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood 110, 441–449 (2007).
Alpdogan, O. et al. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood 105, 865–873 (2005).
Fry, T. J. et al. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood 104, 2794–2800 (2004).
Melchionda, F. et al. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest. 115, 1177–1187 (2005). The first description of the vaccine adjuvant effect of IL-7 and its preferential effects on subdominant antigens.
Frankenberger, B. et al. Influence of CD80, interleukin-2, and interleukin-7 expression in human renal cell carcinoma on the expansion, function, and survival of tumor-specific CTLs. Clin. Cancer Res. 11, 1733–1742 (2005).
Wittig, B. et al. Therapeutic vaccination against metastatic carcinoma by expression-modulated and immunomodified autologous tumor cells: a first clinical phase I/II trial. Hum. Gene Ther. 12, 267–278 (2001).
Kim, T. S., Chung, S. W. & Hwang, S. Y. Augmentation of antitumor immunity by genetically engineered fibroblast cells to express both B7.1 and interleukin-7. Vaccine 18, 2886–2894 (2000).
Colombetti, S., Levy, F. & Chapatte, L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood 113, 6629–6637 (2009).
Nanjappa, S. G., Walent, J. H., Morre, M. & Suresh, M. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J. Clin. Invest. 118, 1027–1039 (2008).
Pellegrini, M. et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144, 601–613 (2011).
Nanjappa, S. G., Kim, E. H. & Suresh, M. Immunotherapeutic effects of IL 7 during a chronic viral infection in mice. Blood 23 Mar 2011 (doi:10.1182/blood-2010-12-323154). References 90 and 91 were the first descriptions of the postive effect of IL-7 on T cell-dependent immunity and viral clearance in chronic infection models.
Unsinger, J. et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 184, 3768–3779 (2010). This study describes the potential for a positive effect of IL-7 therapy in bacterial infection.
Sportes, C. et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205, 1701–1714 (2008). The first complete description of the immunological effects of rhIL-7 therapy in humans, including the important observation that TCR repertoire diversification occurs, at least in part, through the preferential expansion of RTEs and naive T cell populations.
Sportes, C. et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin. Cancer Res. 16, 727–735 (2010).
Beq, S. et al. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood 114, 816–825 (2009). This study shows that glycosylation of IL-7 can decrease its immunogenicity.
Kitazawa, H. et al. IL-7 activates α4β1 integrin in murine thymocytes. J. Immunol. 159, 2259–2264 (1997).
Rosenberg, S. A. et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 29, 313–319 (2006).
Sereti, I. et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113, 6304–6314 (2009). The first report of IL-7 therapy in HIV-infected individuals. In this study, patients were administered a single dose of IL-7, thereby allowing a definitive description of the length of biological effect of IL-7 in humans.
Hakim, F. T. & Gress, R. E. Reconstitution of thymic function after stem cell transplantation in humans. Curr. Opin. Hematol. 9, 490–496 (2002).
Chu, Y. W. et al. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood 104, 1110–1119 (2004).
Levy, Y. et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J. Clin. Invest. 119, 997–1007 (2009). The first report of the effect of multiple doses of IL-7 in HIV-infected individuals.
Patel, A., Patel, J. & Ikwuagwu, J. A case of progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia. J. Antimicrob. Chemother. 65, 2697–2698 (2010).
Abrams, D. et al. Interleukin-2 therapy in patients with HIV infection. N. Engl. J. Med. 361, 1548–1559 (2009).
Shamim, Z. et al. Genetic polymorphisms in the genes encoding human interleukin-7 receptor-α: prognostic significance in allogeneic stem cell transplantation. Bone Marrow Transplant. 37, 485–491 (2006). The first description that polymorphisms in the gene encoding IL-7Rα can contribute to human immune-mediated disease.
Dean, R. M. et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J. Clin. Oncol. 26, 5735–5741 (2008). The first demonstration that serum IL-7 levels are associated with human immune-mediated disease.
Thiant, S. et al. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant. 45, 1546–1552 (2010).
Sinha, M. L., Fry, T. J., Fowler, D. H., Miller, G. & Mackall, C. L. Interleukin 7 worsens graft-versus-host disease. Blood 100, 2642–2649 (2002).
Fewkes, N. M. & Mackall, C. L. Novel γ-chain cytokines as candidate immune modulators in immune therapies for cancer. Cancer J. 16, 392–398 (2010).
Kasten, K. R. et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through γδ T-cell IL-17 production in a murine model of sepsis. Infect. Immun. 78, 4714–4722 (2010).
Uehira, M. et al. The development of dermatitis infiltrated by γδ T cells in IL-7 transgenic mice. Int. Immunol. 5, 1619–1627 (1993).
Lundmark, F. et al. Variation in interleukin 7 receptor α-chain (IL7R) influences risk of multiple sclerosis. Nature Genet. 39, 1108–1113 (2007).
Gregory, S. G. et al. Interleukin 7 receptor α-chain (IL7R) shows allelic and functional association with multiple sclerosis. Nature Genet. 39, 1083–1091 (2007). References 111 and 112 provide definitive evidence that IL-7Rα polymorphisms contribute to the genetic risk of autoimmune disease.
Hartgring, S. A., Bijlsma, J. W., Lafeber, F. P. & van Roon, J. A. Interleukin-7 induced immunopathology in arthritis. Ann. Rheum. Dis. 65 (Suppl. 3), 69–74 (2006).
Hartgring, S. A. et al. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis Rheum. 62, 2716–2725 (2010).
Totsuka, T. et al. IL-7 is essential for the development and the persistence of chronic colitis. J. Immunol. 178, 4737–4748 (2007).
Yamazaki, M. et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J. Immunol. 171, 1556–1563 (2003).
Kirkwood, J. M. et al. Next generation of immunotherapy for melanoma. J. Clin. Oncol. 26, 3445–3455 (2008).
Casadevall, N. et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346, 469–475 (2002).
van de Pavert, S. A. & Mebius, R. E. New insights into the development of lymphoid tissues. Nature Rev. Immunol. 10, 664–674 (2010).
Mebius, R. E. et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+CD4+CD3− cells, as well as macrophages. J. Immunol. 166, 6593–6601 (2001).
Boos, M. D., Yokota, Y., Eberl, G. & Kee, B. L. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 204, 1119–1130 (2007).
GeurtsvanKessel, C. H. et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 206, 2339–2349 (2009).
Rangel-Moreno, J., Moyron-Quiroz, J. E., Hartson, L., Kusser, K. & Randall, T. D. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc. Natl Acad. Sci. USA 104, 10577–10582 (2007).
Lee, Y. et al. Recruitment and activation of naive T cells in the islets by lymphotoxin-β receptor-dependent tertiary lymphoid structure. Immunity 25, 499–509 (2006).
Shields, J. D., Kourtis, I. C., Tomei, A. A., Roberts, J. M. & Swartz, M. A. Induction of lymphoid-like stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Hughes, T. et al. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH17 cytokine interleukin-22. Blood 113, 4008–4010 (2009).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nature Immunol. 10, 75–82 (2009).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Piketty, C. et al. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. J. Infect. Dis. 183, 1328–1335 (2001).
Engsig, F. N. et al. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: a cohort study. BMC Infect. Dis. 10, 318 (2010).
Gutierrez, F. et al. Patients' characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Curr. HIV Res. 6, 100–107 (2008).
Mackall, C. et al. Background to hematopoietic cell transplantation, including post transplant immune recovery. Bone Marrow Transplant. 44, 457–462 (2009).
Acknowledgements
We would like to thank S. Durum for his critical review of the manuscript and his helpful discussions. This work was supported by the Intramural Research Program of the National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Janus kinase–signal transducer and activator of transcription pathway
-
(JAK–STAT pathway). An evolutionarily conserved signalling pathway that is associated with type I and type II cytokines. Receptor ligation by these cytokines leads to a series of events that includes the recruitment and activation of JAKs and the phosphorylation of various STATs, which in turn translocate to the nucleus where they transactivate various genes involved in cell differentiation, survival, apoptosis and proliferation.
- Severe combined immunodeficiency
-
(SCID). A primary (inherited) immunodeficiency characterized by defects in cell-mediated and humoral immune responses. Affected infants commonly die within the first year of life owing to recurrent infections. Mutations in approximately ten different genes have been described to cause this condition, but defects in the common cytokine receptor γ-chain (γc) are the most common and result in X-linked SCID. Other genes that are mutated in patients with SCID include those encoding Janus kinase 3 (JAK3), recombination activating gene 1 (RAG1) and RAG2, IL-7 receptor α-chain (IL-7Rα) and adenosine deaminase.
- pro-B cell
-
A cell at the earliest stage of B cell development in the bone marrow. These cells are characterized by incomplete immunoglobulin heavy-chain gene rearrangement and are defined as being CD19+ cytoplasmic IgM− or, sometimes, as B220+CD43+ (by the Hardy classification scheme).
- pre-B cell
-
A cell at a stage of B cell development in the bone marrow that is characterized by complete immunoglobulin heavy-chain gene rearrangement in the absence of immunoglobulin light-chain gene rearrangement. These cells express the pre-B cell receptor, which comprises a pseudo light chain and a heavy chain. They are phenotypically CD19+ cytoplasmic IgM+ or are sometimes defined as being B220+CD43− cell surface IgM− (by the Hardy classification scheme).
- Sepsis
-
A systemic response to severe infection or tissue damage, leading to a hyperactive and unbalanced network of pro-inflammatory mediators. Vascular permeability, cardiac function and metabolic balance are affected, resulting in tissue necrosis, multi-organ failure and death.
- Delayed-type hypersensitivity
-
(DTH). A cellular immune response to antigen that develops over ∼24–72 hours with the infiltration of T cells and monocytes, and is dependent on the production of T helper 1 cell-specific cytokines.
Rights and permissions
About this article
Cite this article
Mackall, C., Fry, T. & Gress, R. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11, 330–342 (2011). https://doi.org/10.1038/nri2970
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2970
This article is cited by
-
COVID-19 immune signatures in Uganda persist in HIV co-infection and diverge by pandemic phase
Nature Communications (2024)
-
New insights into the stemness of adoptively transferred T cells by γc family cytokines
Cell Communication and Signaling (2023)
-
Extracellular matrix remodelling in dental pulp tissue of carious human teeth through the prism of single-cell RNA sequencing
International Journal of Oral Science (2023)
-
Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting
Nature Communications (2023)
-
Interleukin-7 regulates CD127 expression and promotes CD8+ T cell activity in patients with primary cutaneous melanoma
BMC Immunology (2022)