Key Points
-
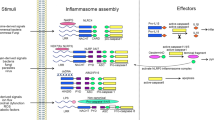
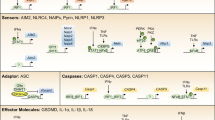
For the known inflammasomes, new cofactors such as caspase 11 and the NAIPs (NLR family, apoptosis inhibitory proteins) have been described. In addition, inflammasome-independent pathways for the processing of interleukin-1β (IL-1β), such as caspase 8 activation, have recently been described.
-
Cell-extrinsic signalling can regulate inflammasome activation. Signalling by pattern recognition or cytokine receptors primes the cell and induces NLRP3 (NOD-, LRR- and pyrin domain-containing 3) and pro-IL-1β expression, whereas signalling by type I interferons and activated T cells reduces inflammasome activation.
-
Energy levels, mitochondrial health and lysosomal compartmentalization are constantly under the surveillance of cellular health sensors such as the apoptosome and inflammasomes. These signalling platforms detect changes in cellular homeostasis and share many structural and functional similarities.
-
NLRP3 is strongly regulated by fluxes of K+, Cl− and Ca2+. In addition, reactive oxygen species, autophagy and endoplasmic reticulum stress are important modulators of NLRP3 activity.
-
The inflammasomes are regulated by pyrin domain- or CARD (caspase activation and recruitment domain)-only proteins, which sequester the signalling molecules. Other proteins that are known to regulate apoptosis also have a role in inflammasome signalling.
-
Understanding the regulatory mechanisms of inflammasome activation will facilitate the development of new classes of drugs that target the inflammasomes.
Abstract
Inflammasomes are key signalling platforms that detect pathogenic microorganisms and sterile stressors, and that activate the highly pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18. In this Review, we discuss the complex regulatory mechanisms that facilitate a balanced but effective inflammasome-mediated immune response, and we highlight the similarities to another molecular signalling platform — the apoptosome — that monitors cellular health. Extracellular regulatory mechanisms are discussed, as well as the intracellular control of inflammasome assembly, for example, via ion fluxes, free radicals and autophagy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Kato, H., Takahasi, K. & Fujita, T. RIG-I-like receptors: cytoplasmic sensors for non-self RNA. Immunol. Rev. 243, 91–98 (2011).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002).
Miao, E. A., Rajan, J. V. & Aderem, A. Caspase-1- induced pyroptotic cell death. Immunol. Rev. 243, 206–214 (2011).
Vajjhala, P. R., Mirams, R. E. & Hill, J. M. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J. Biol. Chem. 287, 41732–41743 (2012).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Proell, M., Gerlic, M., Mace, P. D., Reed, J. C. & Riedl, S. J. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem. J. 449, 613–621 (2013).
Shao, W., Yeretssian, G., Doiron, K., Hussain, S. N. & Saleh, M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282, 36321–36329 (2007).
Thornberry, N. A. et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356, 768–774 (1992).
Gu, Y. et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275, 206–209 (1997).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Ting, J. P.-Y. et al. The NLR gene family: a standard nomenclature. Immunity 28, 285–287 (2008).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 (2007).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunol. 7, 569–575 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin-1β in Salmonella-infected macrophages. Nature Immunol. 7, 576–582 (2006).
Geddes, B. J. et al. Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem. Biophys. Res. Commun. 284, 77–82 (2001).
Hornung, V. & Latz, E. Intracellular DNA recognition. Nature Rev. Immunol. 10, 123–130 (2010).
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nature Immunol. 11, 63–69 (2010).
Arthur, J. C. et al. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J. Immunol. 185, 4515–4519 (2010).
Allen, I. C. et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36, 742–754 (2012).
Anand, P. K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nature Immunol. 11, 997–1004 (2010).
Horvath, G. L., Schrum, J. E., De Nardo, C. M. & Latz, E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol. Rev. 243, 119–135 (2011).
Kerur, N. et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375 (2011).
Abdelaziz, D. H., Amr, K. & Amer, A. O. Nlrc4/Ipaf/CLAN/CARD12: more than a flagellin sensor. Int. J. Biochem. Cell Biol. 42, 789–791 (2010).
Boyden, E. D. & Dietrich, W. F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature Genet. 38, 240–244 (2006).
Khare, S. et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36, 464–476 (2012).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Chen, G. Y., Liu, M., Wang, F., Bertin, J. & Nunez, G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186, 7187–7194 (2011).
Vladimer, G. I. et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37, 96–107 (2012).
Hsu, L.-C. et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl Acad. Sci. USA 105, 7803–7808 (2008).
Ferwerda, G. et al. Engagement of NOD2 has a dual effect on proIL-1β mRNA transcription and secretion of bioactive IL-1β. Eur. J. Immunol. 38, 184–191 (2008).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011). References 34 and 35 demonstrate that NAIPs are crucial for ligand sensing and for the activation of the NLRC4 inflammasome.
Vinzing, M. et al. NAIP and Ipaf control Legionella pneumophila replication in human cells. J. Immunol. 180, 6808–6815 (2008).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Franchi, L., Eigenbrod, T. & Núñez, G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 183, 792–796 (2009).
Shenoy, A. R. et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336, 481–485 (2012). This study is the first description of a regulator of NLRP3 that discriminates between crystalline and non-crystalline ligands.
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). This reference shows the importance of caspase 11 as a cofactor of NLRP3 inflammasome activation in response to Gram-positive bacteria.
Rathinam, V. A. et al. TRIF licenses caspase-11- dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Gurung, P. et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483 (2012).
Sander, L. E. et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474, 385–389 (2011).
Akhter, A. et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37, 35–47 (2012).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Broz, P. & Monack, D. M. Noncanonical inflammasomes: caspase-11 activation and effector mechanisms. PLoS Pathog. 9, e1003144 (2013).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
Provoost, S. et al. NLRP3/caspase-1-independent IL-1β production mediates diesel exhaust particle-induced pulmonary inflammation. J. Immunol. 187, 3331–3337 (2011).
Kono, H., Orlowski, G. M., Patel, Z. & Rock, K. L. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J. Immunol. 189, 3734–3740 (2012).
Gross, O. et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 (2012).
Gringhuis, S. I. et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nature Immunol. 13, 246–254 (2012).
Gross, O. et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436 (2009).
Maelfait, J. et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J. Exp. Med. 205, 1967–1973 (2008).
Vince, J. E. et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 (2012).
Kang, T. B., Yang, S. H., Toth, B., Kovalenko, A. & Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 (2013).
Labbe, K., McIntire, C. R., Doiron, K., Leblanc, P. M. & Saleh, M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity 35, 897–907 (2011).
Miwa, K. et al. Caspase 1-independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nature Med. 4, 1287–1292 (1998).
Bossaller, L. et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 189, 5508–5512 (2012).
Lech, M., Avila-Ferrufino, A., Skuginna, V., Susanti, H. E. & Anders, H. J. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int. Immunol. 22, 717–728 (2010).
Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 (2012).
Py, B. F., Kim, M. S., Vakifahmetoglu-Norberg, H. & Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell 49, 331–338 (2013).
Schroder, K. et al. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology 217, 1325–1329 (2012).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature Immunol. 10, 266–272 (2009).
DeYoung, K. L. et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15, 453–457 (1997).
Qu, Y. et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490, 539–542 (2012). This study decribes the importance of a pathogen-induced post-translational modification event for the activation of NLRC4.
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nature Immunol. 9, 857–865 (2008).
Anderson, J. P. et al. Initial description of the human NLRP3 promoter. Genes Immun. 9, 721–726 (2008).
Guarda, G. et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature 460, 269–273 (2009).
Mishra, B. B. et al. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nature Immunol. 14, 52–60 (2013).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Hernandez-Cuellar, E. et al. Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J. Immunol. 189, 5113–5117 (2012).
Haneklaus, M. et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J. Immunol. 189, 3795–3799 (2012).
Bauernfeind, F. et al. NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 189, 4175–4181 (2012).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunol. 9, 847–856 (2008).
Cain, K., Langlais, C., Sun, X. M., Brown, D. G. & Cohen, G. M. Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. J. Biol. Chem. 276, 41985–41990 (2001).
Purring-Koch, C. & McLendon, G. Cytochrome c binding to Apaf-1: the effects of dATP and ionic strength. Proc. Natl Acad. Sci. USA 97, 11928–11931 (2000).
Pétrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007). This study describes the role of K+ in NLRP1 and NLRP3 activation.
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature Immunol. 11, 385–393 (2010).
Arlehamn, C. S., Petrilli, V., Gross, O., Tschopp, J. & Evans, T. J. The role of potassium in inflammasome activation by bacteria. J. Biol. Chem. 285, 10508–10518 (2010).
Pelegrin, P. & Surprenant, A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1β release through a dye uptake-independent pathway. J. Biol. Chem. 282, 2386–2394 (2007).
Pelegrin, P. & Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 25, 5071–5082 (2006).
Locovei, S., Wang, J. & Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580, 239–244 (2006).
Qu, Y. et al. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186, 6553–6561 (2011).
Compan, V. et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37, 487–500 (2012).
Gong, Y. N. et al. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res. 20, 1289–1305 (2010).
Zhong, Z. et al. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nature Commun. 4, 1611 (2013). This study links Ca2+ mobilization to oxidative stress, both of which are important factors in NLRP3 activation.
Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 109, 11282–11287 (2012).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Lee, G. S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127 (2012).
Rossol, M. et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nature Commun. 3, 1329 (2012). References 91 and 92 describe that GPCRs, which sense extracellular Ca2+, activate the NLRP3 inflammasome by activating phospholipase C and by inhibiting adenylyl cyclase.
Zuo, Y. et al. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf-1. Cell Res. 19, 449–457 (2009).
Zech, B., Kohl, R., von Knethen, A. & Brune, B. Nitric oxide donors inhibit formation of the Apaf-1/caspase-9 apoptosome and activation of caspases. Biochem. J. 371, 1055–1064 (2003).
Kim, Y. M., Talanian, R. V., Li, J. & Billiar, T. R. Nitric oxide prevents IL-1β and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β-converting enzyme). J. Immunol. 161, 4122–4128 (1998).
Cruz, C. M. et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871–2879 (2007).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Cassel, S. L. et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl Acad. Sci. USA 105, 9035–9040 (2008).
van de Veerdonk, F. L. et al. Reactive oxygen species-independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl Acad. Sci. USA 107, 3030–3033 (2010).
Meissner, F. et al. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116, 1570–1573 (2010).
van Bruggen, R. et al. Human NLRP3 inflammasome activation is Nox1–4 independent. Blood 115, 5398–5400 (2010).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Bauernfeind, F. et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187, 613–617 (2011).
Gordy, C. & He, Y. W. The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell 3, 17–27 (2012).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 (2008). This is the first study to describe that autophagy limits IL-1β production.
Shi, C. S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nature Immunol. 13, 255–263 (2012).
Harris, J. et al. Autophagy controls IL-1β secretion by targeting pro-IL-1β for degradation. J. Biol. Chem. 286, 9587–9597 (2011).
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Pathan, N. et al. TUCAN, an antiapoptotic caspase-associated recruitment domain family protein overexpressed in cancer. J. Biol. Chem. 276, 32220–32229 (2001).
Saleh, M. et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440, 1064–1068 (2006).
Saleh, M. et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429, 75–79 (2004).
Stehlik, C. & Dorfleutner, A. COPs and POPs: modulators of inflammasome activity. J. Immunol. 179, 7993–7998 (2007).
Druilhe, A. Srinivasula, S. M., Razmara, M., Ahmad, M. & Alnemri, E. S. Regulation of IL-1β generation by pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 8, 649–657 (2001).
Lamkanfi, M. et al. INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1β generation. J. Biol. Chem. 279, 51729–51738 (2004).
Stehlik, C. et al. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-κB and pro-caspase-1 regulation. Biochem. J. 373, 101–113 (2003).
Dorfleutner, A. et al. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect. Immun. 75, 1484–1492 (2007).
Bedoya, F., Sandler, L. L. & Harton, J. A. Pyrin-only protein 2 modulates NF-κB and disrupts ASC:CLR interactions. J. Immunol. 178, 3837–3845 (2007).
Bryan, N. B. et al. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J. Inflamm. 7, 23 (2010).
Imamura, R. et al. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J. Immunol. 184, 5874–5884 (2010).
Wang, Y. et al. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. Int. Immunol. 16, 777–786 (2004).
Eisenbarth, S. C. et al. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature 484, 510–513 (2012).
Joly, S. et al. Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. J. Immunol. 189, 4713–4717 (2012).
Kinoshita, T., Wang, Y., Hasegawa, M., Imamura, R. & Suda, T. PYPAF3, a PYRIN-containing APAF-1-like protein, is a feedback regulator of caspase-1- dependent interleukin-1β secretion. J. Biol. Chem. 280, 21720–21725 (2005).
Hesker, P. R., Nguyen, M., Kovarova, M., Ting, J. P. & Koller, B. H. Genetic loss of murine pyrin, the familial Mediterranean fever protein, increases interleukin-1β levels. PLoS ONE 7, e51105 (2012).
Chae, J. J. et al. Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity 34, 755–768 (2011).
Vyleta, M. L., Wong, J. & Magun, B. E. Suppression of ribosomal function triggers innate immune signaling through activation of the NLRP3 inflammasome. PLoS ONE 7, e36044 (2012).
Lu, B. et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488, 670–674 (2012).
He, Y., Franchi, L. & Nunez, G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur. J. Immunol. 43, 1147–1152 (2013).
Mayor, A., Martinon, F., De Smedt, T., Petrilli, V. & Tschopp, J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nature Immunol. 8, 497–503 (2007).
Jin, T. et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–571 (2012). This is the first crystal structure of an inflammasome sensor with its ligand.
D'Osualdo, A. et al. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PLoS ONE 6, e27396 (2011).
Frew, B. C., Joag, V. R. & Mogridge, J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 8, e1002659 (2012).
Finger, J. N. et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 287, 25030–25037 (2012).
Martin, A. G. & Fearnhead, H. O. Apocytochrome c blocks caspase-9 activation and Bax-induced apoptosis. J. Biol. Chem. 277, 50834–50841 (2002).
Nakagawa, H. et al. Nitration of specific tyrosine residues of cytochrome c is associated with caspase-cascade inactivation. Biol. Pharm. Bull. 30, 15–20 (2007).
Levinsohn, J. L. et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638 (2012).
Acehan, D. et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9, 423–432 (2002).
Llambi, F. & Green, D. R. Apoptosis and oncogenesis: give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 21, 12–20 (2011).
Bruey, J. M. et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 129, 45–56 (2007).
Faustin, B. et al. Mechanism of Bcl-2 and Bcl-XL inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc. Natl Acad. Sci. USA 106, 3935–3940 (2009).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Feldman, J. L., Dittenhafer-Reed, K. E. & Denu, J. M. Sirtuin catalysis and regulation. J. Biol. Chem. 287, 42419–42427 (2012).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nature Immunol. 14, 454–460 (2013).
Subramanian, N., Natarajan, K., Clatworthy, M. R., Wang, Z. & Germain, R. N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153, 348–361 (2013).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Kim, H. E., Du, F., Fang, M. & Wang, X. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc. Natl Acad. Sci. USA 102, 17545–17550 (2005).
Duncan, J. A. et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl Acad. Sci. USA 104, 8041–8046 (2007).
Yuan, S. et al. Structure of an apoptosome-procaspase-9 CARD complex. Structure 18, 571–583 (2010).
Qi, S. et al. Crystal structure of the Caenorhabditis elegans apoptosome reveals an octameric assembly of CED-4. Cell 141, 446–457 (2010).
Jha, S. et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J. Neurosci. 30, 15811–15820 (2010).
Inoue, M. et al. Interferon-β therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci. Signal. 5, ra38 (2012).
Lamkanfi, M. et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Juliana, C. et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802 (2010).
Acknowledgements
The authors would like to thank C. M. De Nardo and B. G. Monks for critical reading of the manuscript. This work was supported by grants from the US National Institutes of Health (NIH) and the Deutsche Forschungsgemeinschaft (DFG), Germany (to E.L.), and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH, USA (to T.S.X.). E.L. is a member of the excellence cluster ImmunoSensation in Bonn, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- ASC
-
An adaptor protein that was originally found to form protein precipitates in apoptotic cells that are termed protein specks.
- Pyroptosis
-
A rapid form of cell death following caspase 1 activation, which shares characteristics with both apoptosis (such as DNA fragmentation) and necrosis (such as cell swelling and rupture).
- Apoptosome
-
A large multimeric protein complex of apoptotic protease-activating factor 1 (APAF1) that recognizes cytochrome c release from damaged mitochondria and activates caspase 9.
- Death-fold domains
-
Commonly found in proteins that are involved in cell death pathways and in inflammasomes. The four main death-fold domains — the pyrin domain, caspase activation and recruitment domain (CARD), death domain and death effector domain — associate with each other through homotypic interactions.
- NOD-like receptor
-
(NLR). A protein that contains amino-terminal pyrin, caspase-recruitment domains or other signalling domains, followed by a NACHT domain and carboxy-terminal leucine-rich repeats. Some NLR proteins are involved in forming inflammasomes.
- Non-canonical NLRP3 inflammasome
-
An inflammasome-like complex containing NLRP3 (NOD-, LRR- and pyrin domain-containing 3), the adaptor protein ASC, caspase 1 and caspase 11. The term 'non-canonical inflammasome' is used loosely to describe an inflammasome-like complex that does not conform to the three 'canonical' components of a canonical inflammasome: an inflammasome sensor molecule, ASC and caspase 1. Two other non-canonical inflammasomes have also been described so far: one containing dectin 1, MALT1 (mucosa-associated lymphoid tissue lymphoma translocation protein 1), ASC and caspase 8 (termed the non-canonical caspase 8 inflammasome), and a caspase 11-activating platform that has not yet been fully described.
- Ripoptosome
-
A cytosolic multiprotein complex that induces cell death following genotoxic stress or depletion of inhibitor of apoptosis protein (IAP). The core ripoptosome contains receptor-interacting protein 1 (RIP1), FAS-associated death domain protein (FADD) and caspase 8, but it can also recruit other proteins such as RIP3.
- Amyloid-β
-
An endogenous peptide that is generated by proteases in the brain. It is prone to aggregation and plaque formation. Amyloid-β plaques are a hallmark of Alzheimer's disease and can activate the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome.
- Cryopyrin-associated periodic syndrome
-
(CAPS). Characterized by NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome hyperactivity and the excessive release of interleukin-1β, which leads to an autoinflammatory disease phenotype with periodic fever episodes, urticaria and often severe arthritis.
- MicroRNA
-
Small, endogenous RNA molecules that can recruit the RNA-induced silencing complex to an mRNA, leading to inhibition of translation or to degradation of the mRNA.
- Autophagy
-
A homeostatic process during which cellular components are recycled through the lysosomal compartment. Its main triggers include nutrient starvation, defective organelles and infection.
Rights and permissions
About this article
Cite this article
Latz, E., Xiao, T. & Stutz, A. Activation and regulation of the inflammasomes. Nat Rev Immunol 13, 397–411 (2013). https://doi.org/10.1038/nri3452
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3452
This article is cited by
-
A novel complement C3 inhibitor CP40-KK protects against experimental pulmonary arterial hypertension via an inflammasome NLRP3 associated pathway
Journal of Translational Medicine (2024)
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Leukocyte differential gene expression prognostic value for high versus low seizure frequency in temporal lobe epilepsy
BMC Neurology (2024)
-
Inflammasomes in neurological disorders — mechanisms and therapeutic potential
Nature Reviews Neurology (2024)
-
DSPE-PEG2000-methotrexate nanoparticles encapsulating phenobarbital sodium kill cancer cells by inducing pyroptosis
Journal of Molecular Medicine (2024)