Key Points
-
Bacteria have evolved a multitude of strategies for manipulating and exploiting the host to establish an infection and replicate, while keeping the host cells, and thus their replicative niche, alive.
-
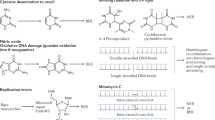
Bacterial infections can cause DNA damage in host cells, either directly by secreting genotoxic proteins or through mechanisms involving host response to the infection.
-
Some bacterial species simultaneously disrupt the host's DNA damage response through multiple pathways. This can lead to genomic instability and, in consequence, tumorigenic transformation.
-
The environment established upon infection may promote the escape of such transformed cells from tumour surveillance mechanisms.
-
Chronic infections that evade the immune system seem to be particularly dangerous in this context, and several of them, such as chronic infections by Helicobacter pylori, Chlamydia trachomatis and Salmonella enterica subsp. enterica serovar Typhi, have been associated with the development of human cancers.
Abstract
Mammalian cells possess sophisticated genome surveillance and repair mechanisms, executed by the so-called DNA damage response (DDR), failure of which leads to accumulation of DNA damage and genomic instability. Mounting evidence suggests that bacterial infections can elicit DNA damage in host cells, and certain pathogens induce such damage as part of their multi-faceted infection programme. Bacteria-mediated DNA damage can occur either directly through the formation of toxins with genotoxic activities or indirectly as a result of the activation of cell-autonomous or immune defence mechanisms against the pathogen. Moreover, host-cell signalling routes involved in the DDR can be altered in response to an infection, and this, in the context of DNA damage elicited by the pathogen, has the potential to trigger mutations and cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lindahl, T. & Barnes, D. E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 65, 127–133 (2000).
Ciccia, A. & Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 (2010).
Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001).
IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 61, 1–241 (1994).
Azenabor, A. A. & Mahony, J. B. Generation of reactive oxygen species and formation and membrane lipid peroxides in cells infected with Chlamydia trachomatis. Int. J. Infect. Dis. 4, 46–50 (2000).
Wu, M. et al. Host DNA repair proteins in response to Pseudomonas aeruginosa in lung epithelial cells and in mice. Infect. Immun. 79, 75–87 (2011).
Hardbower, D. M., de Sablet, T., Chaturvedi, R. & Wilson, K. T. Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes 4, 475–481 (2013).
Abdul-Sater, A. A. et al. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J. Biol. Chem. 285, 41637–41645 (2010).
Chumduri, C., Gurumurthy, R. K., Zadora, P. K., Mi, Y. & Meyer, T. F. Chlamydia infection promotes host DNA damage and proliferation but impairs the DNA damage response. Cell Host Microbe 13, 746–758 (2013). This study shows that C. trachomatis induces persistent DSBs in infected cells and simultaneously suppresses recruitment of ATM and 53BP1, while maintaining cells in a proliferative state.
Tattoli, I. et al. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-κB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 9, 293–300 (2008).
Lam, G. Y. et al. Listeriolysin O suppresses phospholipase C-mediated activation of the microbicidal NADPH oxidase to promote Listeria monocytogenes infection. Cell Host Microbe 10, 627–634 (2011).
Leitao, E. et al. Listeria monocytogenes induces host DNA damage and delays the host cell cycle to promote infection. Cell Cycle 13, 928–940 (2014).
Samba-Louaka, A. et al. Listeria monocytogenes dampens the DNA damage response. PLoS Pathog. 10, e1004470 (2014). This paper shows that the L. monocytogenes toxin LLO degrades the DDR sensor MRE11, despite DSB induction by the bacteria, and that this repression of the DDR promotes infection.
Plotkowski, M. C. et al. Early mitochondrial dysfunction, superoxide anion production, and DNA degradation are associated with non-apoptotic death of human airway epithelial cells induced by Pseudomonas aeruginosa exotoxin A. Am. J. Respir. Cell. Mol. Biol. 26, 617–626 (2002).
Saliba, A. M. et al. Implications of oxidative stress in the cytotoxicity of Pseudomonas aeruginosa ExoU. Microbes Infect. 8, 450–459 (2006).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial ROS-induced ROS release: an update and review. Biochim. Biophys. Acta 1757, 509–517 (2006).
Wei, L. & Dirksen, R. T. Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondrial superoxide flashes: from discovery to new controversies. J. Gen. Physiol. 139, 425–434 (2012).
Roy, A. et al. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol. Pharmacol. 74, 1292–1307 (2008).
Rai, P. et al. Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proc. Natl Acad. Sci. USA 112, E3421–E3430 (2015).
Chaturvedi, R. et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141, 1696–1708.e2 (2011).
Kim, J. M. et al. Vacuolating cytotoxin in Helicobacter pylori water-soluble proteins upregulates chemokine expression in human eosinophils via Ca2+ influx, mitochondrial reactive oxygen intermediates, and NF-κB activation. Infect. Immun. 75, 3373–3381 (2007).
Gong, M., Ling, S. S., Lui, S. Y., Yeoh, K. G. & Ho, B. Helicobacter pylori γ-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease. Gastroenterology 139, 564–573 (2010).
Uberti, A. F. et al. Pro-inflammatory properties and neutrophil activation by Helicobacter pylori urease. Toxicon 69, 240–249 (2013).
Wang, G., Hong, Y., Olczak, A., Maier, S. E. & Maier, R. J. Dual roles of Helicobacter pylori NapA in inducing and combating oxidative stress. Infect. Immun. 74, 6839–6846 (2006).
Gorlach, A., Bertram, K., Hudecova, S. & Krizanova, O. Calcium and ROS: a mutual interplay. Redox Biol. 6, 260–271 (2015).
Ermak, G. & Davies, K. J. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 38, 713–721 (2002).
Raza, Y. et al. Oxidative DNA damage as a potential early biomarker of Helicobacter pylori associated carcinogenesis. Pathol. Oncol. Res. 20, 839–846 (2014).
Kidane, D., Murphy, D. L. & Sweasy, J. B. Accumulation of abasic sites induces genomic instability in normal human gastric epithelial cells during Helicobacter pylori infection. Oncogenesis 3, e128 (2014). This report shows that DNA damage induced by H. pylori leads to the accumulation of abasic sites, and that BER processing of these sites generates DSBs.
Toller, I. M. et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc. Natl Acad. Sci. USA 108, 14944–14949 (2011). This paper finds that direct cell contact is sufficient for the induction of DSBs in H. pylori -infected cells and that prolonged infection leads to persistent unrepaired breaks.
Hartung, M. L. et al. H. pylori-induced DNA strand breaks are introduced by nucleotide excision repair endonucleases and promote NF-κB target gene expression. Cell Rep. 13, 70–79 (2015). This study reports that NER endonucleases induce DSB breaks in H. pylori -infected cells, which lead to increased NF-κB target gene activation, thus promoting the survival of host cells.
Matsumoto, Y. et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat. Med. 13, 470–476 (2007).
Shimizu, T. et al. Accumulation of somatic mutations in TP53 in gastric epithelium with Helicobacter pylori infection. Gastroenterology 147, 407–417.e3 (2014).
Mechtcheriakova, D., Svoboda, M., Meshcheryakova, A. & Jensen-Jarolim, E. Activation-induced cytidine deaminase (AID) linking immunity, chronic inflammation, and cancer. Cancer Immunol. Immunother. 61, 1591–1598 (2012).
Liu, M. et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature 451, 841–845 (2008).
Gurumurthy, R. K. et al. Dynamin-mediated lipid acquisition is essential for Chlamydia trachomatis development. Mol. Microbiol. 94, 186–201 (2014).
Elwell, C., Mirrashidi, K. & Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 14, 385–400 (2016).
Zahlten, J. et al. Streptococcus pneumoniae-induced oxidative stress in lung epithelial cells depends on pneumococcal autolysis and is reversible by resveratrol. J. Infect. Dis. 211, 1822–1830 (2015).
Oosthuysen, W. F., Mueller, T., Dittrich, M. T. & Schubert-Unkmeir, A. Neisseria meningitidis causes cell cycle arrest of human brain microvascular endothelial cells at S phase via p21 and cyclin G2. Cell. Microbiol. 18, 46–65 (2016).
Grasso, F. & Frisan, T. Bacterial genotoxins: merging the DNA damage response into infection biology. Biomolecules 5, 1762–1782 (2015).
Homburg, S., Oswald, E., Hacker, J. & Dobrindt, U. Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli. FEMS Microbiol. Lett. 275, 255–262 (2007).
Nougayrede, J. P. et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851 (2006). This study identified the genotoxic activity of the colibactin biosynthetic pathway encoded by the pks genomic island of E. coli.
Putze, J. et al. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun. 77, 4696–4703 (2009).
Vizcaino, M. I. & Crawford, J. M. The colibactin warhead crosslinks DNA. Nat. Chem. 7, 411–417 (2015).
Guidi, R. et al. Salmonella enterica delivers its genotoxin through outer membrane vesicles secreted from infected cells. Cell. Microbiol. 15, 2034–2050 (2013).
Bezine, E., Vignard, J. & Mirey, G. The cytolethal distending toxin effects on mammalian cells: a DNA damage perspective. Cells 3, 592–615 (2014).
DiRienzo, J. M. Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins (Basel) 6, 3098–3116 (2014).
Fedor, Y. et al. From single-strand breaks to double-strand breaks during S-phase: a new mode of action of the Escherichia coli cytolethal distending toxin. Cell. Microbiol. 15, 1–15 (2013).
Sandvig, K. Shiga toxins. Toxicon 39, 1629–1635 (2001).
Johannes, L. & Romer, W. Shiga toxins — from cell biology to biomedical applications. Nat. Rev. Microbiol. 8, 105–116 (2010).
Brigotti, M. et al. Shiga toxin 1: damage to DNA in vitro. Toxicon 39, 341–348 (2001).
Brigotti, M. et al. Damage to nuclear DNA induced by Shiga toxin 1 and ricin in human endothelial cells. FASEB J. 16, 365–372 (2002).
Bergounioux, J. et al. Calpain activation by the Shigella flexneri effector VirA regulates key steps in the formation and life of the bacterium's epithelial niche. Cell Host Microbe 11, 240–252 (2012).
Vielfort, K. et al. Neisseria gonorrhoeae infection causes DNA damage and affects the expression of p21, 27 and p53 in non-tumor epithelial cells. J. Cell Sci. 126, 339–347 (2013).
Weyler, L. et al. Restriction endonucleases from invasive Neisseria gonorrhoeae cause double-strand breaks and distort mitosis in epithelial cells during infection. PLoS ONE 9, e114208 (2014).
Guerra, L. et al. Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage. PLoS ONE 5, e8924 (2010).
Hassane, D. C., Lee, R. B. & Pickett, C. L. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect. Immun. 71, 541–545 (2003).
Li, L. et al. The Haemophilus ducreyi cytolethal distending toxin activates sensors of DNA damage and repair complexes in proliferating and non-proliferating cells. Cell. Microbiol. 4, 87–99 (2002).
Alaoui-El-Azher, M. et al. Role of the ATM-checkpoint kinase 2 pathway in CDT-mediated apoptosis of gingival epithelial cells. PLoS ONE 5, e11714 (2010).
Fahrer, J. et al. Cytolethal distending toxin (CDT) is a radiomimetic agent and induces persistent levels of DNA double-strand breaks in human fibroblasts. DNA Repair (Amst.) 18, 31–43 (2014).
Blazkova, H. et al. Bacterial intoxication evokes cellular senescence with persistent DNA damage and cytokine signalling. J. Cell. Mol. Med. 14, 357–367 (2010).
Cortes-Bratti, X., Frisan, T. & Thelestam, M. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon 39, 1729–1736 (2001).
Cortes-Bratti, X., Karlsson, C., Lagergard, T., Thelestam, M. & Frisan, T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 276, 5296–5302 (2001).
Sato, T. et al. p53-independent expression of p21CIP1/WAF1 in plasmacytic cells during G2 cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect. Immun. 70, 528–534 (2002).
Guidi, R. et al. Chronic exposure to the cytolethal distending toxins of Gram-negative bacteria promotes genomic instability and altered DNA damage response. Cell. Microbiol. 15, 98–113 (2013). This report finds that long-term exposure of cells to sublethal doses of Cdt leads to mutations and chromosomal aberrations while cell survival is maintained by p38 MAPK, leading to the emergence of transformed cells exhibiting anchorage-independent growth.
Guerra, L. et al. A bacterial cytotoxin identifies the RhoA exchange factor Net1 as a key effector in the response to DNA damage. PLoS ONE 3, e2254 (2008).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Fernet, M., Megnin-Chanet, F., Hall, J. & Favaudon, V. Control of the G2/M checkpoints after exposure to low doses of ionising radiation: implications for hyper-radiosensitivity. DNA Repair (Amst.) 9, 48–57 (2010).
Cuevas-Ramos, G. et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl Acad. Sci. USA 107, 11537–11542 (2010). This paper reports that infection of enterocytes with low doses of colibactin-positive E. coli leads to DSBs that are not completely repaired, leading to chromosomal instability and the emergence of anchorage-independent growth.
Talukder, K. A. et al. Activation of p53/ATM-dependent DNA damage signaling pathway by shiga toxin in mammalian cells. Microb. Pathog. 52, 311–317 (2012).
Machado, A. M. et al. Helicobacter pylori infection induces genetic instability of nuclear and mitochondrial DNA in gastric cells. Clin. Cancer Res. 15, 2995–3002 (2009).
Sepulveda, A. R. et al. CpG methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with Helicobacter pylori infection. Gastroenterology 138, 1836–1844 (2010).
Kim, J. J. et al. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology 123, 542–553 (2002).
Park, D. I. et al. Effect of Helicobacter pylori infection on the expression of DNA mismatch repair protein. Helicobacter 10, 179–184 (2005).
Hanada, K. et al. Helicobacter pylori infection introduces DNA double-strand breaks in host cells. Infect. Immun. 82, 4182–4189 (2014).
Koeppel, M., Garcia-Alcalde, F., Glowinski, F., Schlaermann, P. & Meyer, T. F. Helicobacter pylori infection causes characteristic DNA damage patterns in human cells. Cell Rep. 11, 1703–1713 (2015).
Gaillard, H., Garcia-Muse, T. & Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer 15, 276–289 (2015).
Lakin, N. D. & Jackson, S. P. Regulation of p53 in response to DNA damage. Oncogene 18, 7644–7655 (1999).
Buti, L. et al. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc. Natl Acad. Sci. USA 108, 9238–9243 (2011).
Wei, J. et al. Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells. Gastroenterology 139, 1333–1343 (2010).
Bhardwaj, V. et al. Helicobacter pylori bacteria alter the p53 stress response via ERK–HDM2 pathway. Oncotarget 6, 1531–1543 (2015).
Wei, J. et al. Pathogenic bacterium Helicobacter pylori alters the expression profile of p53 protein isoforms and p53 response to cellular stresses. Proc. Natl Acad. Sci. USA 109, E2543–E2550 (2012).
Wei, J., Zaika, E. & Zaika, A. p53 Family: role of protein isoforms in human cancer. J. Nucleic Acids 2012, 687359 (2012).
Gurumurthy, R. K. et al. A loss-of-function screen reveals Ras- and Raf-independent MEK–ERK signaling during Chlamydia trachomatis infection. Sci. Signal. 3, ra21 (2010).
Di Micco, R. et al. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat. Cell Biol. 13, 292–302 (2011).
Schuster-Bockler, B. & Lehner, B. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature 488, 504–507 (2012).
Cao, X. et al. The use of transformed IMR90 cell model to identify the potential extra-telomeric effects of hTERT in cell migration and DNA damage response. BMC Biochem. 15, 17 (2014).
Padberg, I., Janssen, S. & Meyer, T. F. Chlamydia trachomatis inhibits telomeric DNA damage signaling via transient hTERT upregulation. Int. J. Med. Microbiol. 303, 463–474 (2013).
Gonzalez, E. et al. Chlamydia infection depends on a functional MDM2–p53 axis. Nat. Commun. 5, 5201 (2014).
Siegl, C., Prusty, B. K., Karunakaran, K., Wischhusen, J. & Rudel, T. Tumor suppressor p53 alters host cell metabolism to limit Chlamydia trachomatis infection. Cell Rep. 9, 918–929 (2014). This reference and reference 88 show that C. trachomatis infection depends on depletion of the tumour suppressor p53 in host cells.
Binnicker, M. J., Williams, R. D. & Apicella, M. A. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell. Microbiol. 5, 549–560 (2003).
Chen, A. & Seifert, H. S. Neisseria gonorrhoeae-mediated inhibition of apoptotic signalling in polymorphonuclear leukocytes. Infect. Immun. 79, 4447–4458 (2011).
Parsonnet, J. et al. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325, 1127–1131 (1991).
Uemura, N. et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345, 784–789 (2001).
Escobar-Paramo, P. et al. Large-scale population structure of human commensal Escherichia coli isolates. Appl. Environ. Microbiol. 70, 5698–5700 (2004).
Andia, M. E., Hsing, A. W., Andreotti, G. & Ferreccio, C. Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int. J. Cancer 123, 1411–1416 (2008).
Randi, G., Franceschi, S. & La Vecchia, C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int. J. Cancer 118, 1591–1602 (2006).
Chaturvedi, A. K. et al. Chlamydia pneumoniae infection and risk for lung cancer. Cancer Epidemiol. Biomarkers Prev. 19, 1498–1505 (2010).
Idahl, A. et al. Chlamydia trachomatis and Mycoplasma genitalium plasma antibodies in relation to epithelial ovarian tumors. Infect. Dis. Obstet. Gynecol. 2011, 824627 (2011).
Smith, J. S. et al. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int. J. Cancer 111, 431–439 (2004).
Touati, E. When bacteria become mutagenic and carcinogenic: lessons from H. pylori. Mutat. Res. 703, 66–70 (2010).
Touati, E. et al. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology 124, 1408–1419 (2003).
Wang, K. et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 46, 573–582 (2014).
Matsumoto, Y. et al. Up-regulation of activation-induced cytidine deaminase causes genetic aberrations at the CDKN2b-CDKN2a in gastric cancer. Gastroenterology 139, 1984–1994 (2010).
Endo, Y., Marusawa, H. & Chiba, T. Involvement of activation-induced cytidine deaminase in the development of colitis-associated colorectal cancers. J. Gastroenterol. 46 (Suppl. 1), 6–10 (2011).
Brown, H. M. et al. Multinucleation during C. trachomatis infections is caused by the contribution of two effector pathways. PLoS ONE 9, e100763 (2014).
Knowlton, A. E., Fowler, L. J., Patel, R. K., Wallet, S. M. & Grieshaber, S. S. Chlamydia induces anchorage independence in 3T3 cells and detrimental cytological defects in an infection model. PLoS ONE 8, e54022 (2013).
Secher, T., Samba-Louaka, A., Oswald, E. & Nougayrede, J. P. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS ONE 8, e77157 (2013).
Cougnoux, A. et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63, 1932–1942 (2014).
Dalmasso, G., Cougnoux, A., Delmas, J., Darfeuille-Michaud, A. & Bonnet, R. The bacterial genotoxin colibactin promotes colon tumor growth by modifying the tumor microenvironment. Gut Microbes 5, 675–680 (2014).
Santiago, A., Li, D., Zhao, L. Y., Godsey, A. & Liao, D. p53 SUMOylation promotes its nuclear export by facilitating its release from the nuclear export receptor CRM1. Mol. Biol. Cell 24, 2739–2752 (2013).
Santiago, A., Godsey, A. C., Hossain, J., Zhao, L. Y. & Liao, D. Identification of two independent SUMO-interacting motifs in Daxx: evolutionary conservation from Drosophila to humans and their biochemical functions. Cell Cycle 8, 76–87 (2009).
Siegl, C. & Rudel, T. Modulation of p53 during bacterial infections. Nat. Rev. Microbiol. 13, 741–748 (2015).
Scanu, T. et al. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe 17, 763–774 (2015). This paper shows that manipulation of the AKT and MAPK pathways during S . Typhi infection of primary cells with predisposing mutations can directly lead to transformation.
Boccellato, F. & Meyer, T. F. Bacteria moving into focus of human cancer. Cell Host Microbe 17, 728–730 (2015).
Del Bel Belluz, L. et al. The typhoid toxin promotes host survival and the establishment of a persistent asymptomatic infection. PLoS Pathog. 12, e1005528 (2016).
Hartlova, A. et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity 42, 332–343 (2015).
Barber, G. N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 15, 760–770 (2015).
Uehara, T. et al. H. pylori infection is associated with DNA damage of Lgr5-positive epithelial stem cells in the stomach of patients with gastric cancer. Dig. Dis. Sci. 58, 140–149 (2013).
Sigal, M. et al. Helicobacter pylori activates and expands Lgr5+ stem cells through direct colonization of the gastric glands. Gastroenterology 148, 1392–1404.e21 (2015).
Klein, G. Evolutionary aspects of cancer resistance. Semin. Cancer Biol. 25, 10–14 (2014).
Bartfeld, S. et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136.e6 (2015).
Schlaermann, P. et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 65, 202–213 (2016).
Kryston, T. B., Georgiev, A. B., Pissis, P. & Georgakilas, A. G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 711, 193–201 (2011).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Altieri, F., Grillo, C., Maceroni, M. & Chichiarelli, S. DNA damage and repair: from molecular mechanisms to health implications. Antioxid. Redox Signal. 10, 891–937 (2008).
Nouspikel, T. DNA repair in mammalian cells: Nucleotide excision repair: variations on versatility. Cell. Mol. Life Sci. 66, 994–1009 (2009).
Blackwood, J. K. et al. End-resection at DNA double-strand breaks in the three domains of life. Biochem. Soc. Trans. 41, 314–320 (2013).
Lamarche, B. J., Orazio, N. I. & Weitzman, M. D. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 584, 3682–3695 (2010).
Schlacher, K. et al. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145, 529–542 (2011).
Schlacher, K., Wu, H. & Jasin, M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell 22, 106–116 (2012).
Acknowledgements
The authors thank D. Schad for help with the figures to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Bacterial secretion systems
-
Molecular machineries used to transport bacterial effector molecules to their environment or simultaneously across the host cell membrane.
- NLRX1
-
(Nucleotide-binding oligomerization domain, leucine-rich repeat containing X1 protein). A member of the NOD-like receptor family that translocates to the mitochondria and has been shown to augment reactive oxygen species production from the mitochondria in response to infections.
- Phagosome
-
Membrane-bound organelle that degrades proteins from internalized particles into peptides.
- Nosocomial diseases
-
Diseases caused by hospital-acquired infections, frequently caused by antibiotic-resistant bacteria, posing a high risk to susceptible patients.
- Exotoxins
-
Bacterial secreted toxins that can bind to a cell surface receptor and stimulate intracellular signalling pathways (type I), damage the cell membrane (type II) or exert effects intracellularly (type III), causing damage to the host by destroying cells or disrupting their normal metabolism.
- Bacterial virulence factor
-
Chromosome- or plasmid-encoded molecules, including bacterial toxins that contribute to colonization or pathogenicity.
- CagA
-
(Cytotoxin-associated gene A). A 120–145 kDa protein expressed by Cag pathogenicity island-positive Helicobacter pylori strains. CagA is translocated into host cells and is associated with increased inflammation and risk of gastric cancer.
- Cag pathogenicity island
-
(CagPAI). A 40 kb DNA segment of Helicobacter pylori, encoding 20 genes, including CagA and proteins involved in its translocation to the host cell.
- Somatic hypermutation
-
Mutation of variable immunoglobulin gene regions in immune cells induced by activation-induced cytidine deaminase (AID), which drives B cell diversification and immune adaptation.
- Class switch recombination
-
Programmed removal of sections of the antibody heavy chain locus from B cell chromosomes through generation of double-strand breaks, followed by rejoining of the segments by non-homologous end-joining, leading to generation of a different antibody class.
- Replication fork stalling
-
Pausing (sometimes indefinite) of the progress of the replication fork complex (where DNA unwinding and DNA synthesis take place), owing to the presence of impediments, such as DNA lesions.
- pks genomic island
-
A 54-kb genomic island of Escherichia coli, which encodes several genes that are responsible for the synthesis of the genotoxin colibactin.
- Gram-negative bacteria
-
Bacterial species that do not have a thick cell wall, as detected by the Gram stain for peptidoglycan.
- p38 MAPK
-
A class of MAPKs that respond to stress stimuli and have a major role in apoptosis, differentiation, survival, proliferation, development and inflammation.
- γH2AX
-
Ser139 phosphorylated histone H2AX, which serves as an early cellular response and a sensitive marker for DNA double-strand breaks.
- Anaphase bridges
-
Chromatin bridges formed during anaphase, caused by fusion of telomeres of sister chromatids, leading to failure to completely segregate them into their respective daughter cells.
- Spindle assembly checkpoint
-
A process ensuring accurate chromosome segregation into daughter cells, which acts by delaying cell division during mitosis and meiosis until proper attachment of chromosomes to the microtubule spindle apparatus is achieved.
- Micronuclei
-
Small, extranuclear bodies, resulting from chromosome fragments or whole chromosomes being separated from daughter nuclei during mitosis, either by breaking of an anaphase bridge or by a double-strand break in the DNA.
- Microsatellite instability
-
Hypermutability of short tandem repeat DNA sequences, which results from defects in mismatch repair.
- Senescence-associated secretory phenotype
-
(SASP). Expression of a set of secreted growth factors, inflammatory cytokines, proteases and matrix components by senescent cells, which is associated with pro-malignant changes in surrounding cells.
Rights and permissions
About this article
Cite this article
Chumduri, C., Gurumurthy, R., Zietlow, R. et al. Subversion of host genome integrity by bacterial pathogens. Nat Rev Mol Cell Biol 17, 659–673 (2016). https://doi.org/10.1038/nrm.2016.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2016.100
This article is cited by
-
Biosensors, modern technology for the detection of cancer-associated bacteria
Biotechnology Letters (2022)
-
Taxonomic diversity of sputum microbiome in lung cancer patients and its relationship with chromosomal aberrations in blood lymphocytes
Scientific Reports (2020)
-
Staphylococcus aureus induces DNA damage in host cell
Scientific Reports (2019)
-
Human Papilloma Virus and Chlamydia trachomatis: Casual Acquaintances or Partners in Crime?
Current Clinical Microbiology Reports (2019)