Key Points
-
The gastrointestinal tract presents a continuous secreted and cell surface barrier to potential enteric pathogens. Specialized gastrointestinal epithelial cells secrete large amounts of mucin glycoproteins and antimicrobial molecules that, together, form the mucus barrier to infection. Although the lumen of the gastrointestinal tract contains large numbers of commensal microorganisms, the inner layers of mucus are sterile.
-
Secreted mucins are large, heavily O-glycosylated glycoproteins that are produced by goblet cells. During their biosynthesis, mucins homo-oligomerize into complex polymeric networks that, when secreted, give mucus its viscoelastic properties.
-
Antimicrobial molecules are produced throughout the gastrointestinal tract but particularly by the specialized Paneth cells in the small intestine. These molecules target different classes of pathogens and help keep the inner mucus layer sterile.
-
Cell surface mucins are heavily O-glycosylated transmembrane glycoproteins that are present on the apical surface of all gastrointestinal epithelial cells. These mucins limit binding of pathogens to epithelial cells by steric hindrance and by acting as releasable decoys for microbial adhesins.
-
Deficiencies in secreted or cell surface mucins in animal models lead to increased pathology during infection.
-
Pathogens have evolved multiple strategies to penetrate the mucosal barrier, including: disruption and penetration of the mucus, avoidance of the mucus barrier, and disruption of epithelial integrity and epithelial production of barrier components.
-
The production of components of the mucus barrier is influenced by the normal microbiota and by both innate and adaptive immune responses to pathogens. There are changes in the rate of mucus production and the content of mucus in response to infection; these factors are components of the mechanism of clearance of enteric pathogens and parasites.
Abstract
The extracellular secreted mucus and the cell surface glycocalyx prevent infection by the vast numbers of microorganisms that live in the healthy gut. Mucin glycoproteins are the major component of these barriers. In this Review, we describe the components of the secreted and cell surface mucosal barriers and the evidence that they form an effective barricade against potential pathogens. However, successful enteric pathogens have evolved strategies to circumvent these barriers. We discuss the interactions between enteric pathogens and mucins, and the mechanisms that these pathogens use to disrupt and avoid mucosal barriers. In addition, we describe dynamic alterations in the mucin barrier that are driven by host innate and adaptive immune responses to infection.
Similar content being viewed by others
Main
An enormous surface area of mucosal epithelial cells in the gastrointestinal tract is potentially exposed to enteric microorganisms. The mucosal epithelium has highly specialized functions throughout the gastrointestinal tract to allow ingested food to be digested, absorbed and processed for excretion. These processes must coexist with the need to provide a barrier both to the commensal microorganisms that ferment undigested food material in the lumen of the intestine and to potential viral, bacterial and eukaryotic pathogens. The mutually beneficial relationship between the commensal microorganisms and the host is a delicate balance that is maintained by appropriate host barrier function and by specific adaptations of the microorganisms. Although commensal microorganisms do not generally cause disease, this is context dependent: when the mucosal surface is damaged, the commensal microorganisms can become opportunistic pathogens and contribute to pathology. In order to protect the mucosa, the host produces a thick, complex layer of mucus that covers the gastrointestinal tract in the stomach, the small intestine and the large intestine.
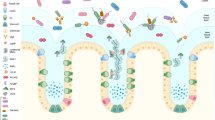
The constituents of this mucus barrier vary throughout the gastrointestinal tract and are rapidly turned over, can respond dynamically to infection, and are regulated by underlying innate and adaptive immunity. The mucus layer is produced by specialized secretory cells that are found throughout the entire intestinal tract (Fig. 1). In the mouth and oesophagus, the mucosa consists of a squamous epithelial layer with underlying glands that secrete the fluid (saliva) which lubricates and protects these tissues. Saliva is a complex fluid containing mucin glycoproteins and antimicrobial molecules, which represent the first host challenge for ingested enteric pathogens. From the stomach to the rectum, the mucosa consists of a single layer of columnar epithelial cells covered by a layer of secreted mucus that is at its thickest in the stomach and colon. This is the major barrier separating the epithelial cells and underlying host tissues from the commensal microbiota. Like saliva, mucus is a complex fluid that is rich in mucin glycoproteins, which give mucus its viscous properties, and a diverse range of antimicrobial molecules, including a broad range of nonspecific molecules and secreted immunoglobulins that are directed against specific microbial antigens. The spatial separation of the colonic commensal microbiota and the epithelium by the mucus layer is strong evidence of the effectiveness of mucus as a barrier1,2. Typically, anaerobic commensal microorganisms occupy only the outer mucus layer, leaving the inner mucus layer effectively sterile1,2. Ongoing degradation of the outer mucus layer by anaerobic bacteria means that the mucus must be continually replaced by the epithelium.
The mucosa consists of a single layer of columnar epithelial cells covered by a layer of secreted mucus. The secreted mucus forms two layers, a thinner inner layer that is sterile and difficult to dislodge and an outer layer that is not sterile and is more easily removed. a | The outer layer of the stomach contains low numbers of transiting bacteria and measures ∼120 μm in rats and mice, whereas the inner layer is sterile and measures ∼100–150 μm. The mucus consists of mucin 5AC (MUC5AC) and MUC6 and is produced by mucus cells (stomach goblet cells). b | The mucus layer is thinner in the small intestine, consisting of an inner layer of ∼15–30 μm and an outer layer of 100–400 μm; it is thickest in the ileum, where there are approximately 105–107 bacteria per gram of faeces in the lumen. Mucus in the small intestine (consisting of mainly MUC2) is produced by goblet cells and Paneth cells. c | Mucus in the large intestine (mainly MUC2) is predominantly produced by goblet cells and consists of a sterile inner layer of ∼100 μm and a thick outer layer of ∼700 μm. Numbers of bacteria are greater in the large intestine (1010–1012 per gram) than in the small intestine owing to low numbers of bacteria in most food, bacterial killing by acid in the stomach, and the fast transit time and high concentration of bile acids in the small intestine, which limit bacterial growth. In the colon, the dwell time is longer, bile acid concentrations are low, endogenous mucus substrate is plentiful and Paneth cells and their secretions are sparse. Therefore, the environment in the colon (which is a fermentative organ analogous to the rumen) encourages a large, complex microbial consortium. Gastrointestinal epithelial cells, particularly those that do not produce mucus, are covered with microvilli containing a high density of transmembrane cell surface mucins.
Enteric pathogens recognize environmental cues within the mucus and have developed a range of strategies to subvert and avoid this barrier to reach the underlying epithelium, where they can initiate disease. The host response to these pathogens includes altering the production rate, constituents and biophysical properties of the mucus. Although the barrier is effective at excluding commensal microorganisms, enteric pathogens and parasites have evolved a wide range of strategies for circumventing it to cause pathology in the host. Both innate and adaptive immune responses modulate and contribute to the barrier to commensal microorganisms, and can respond rapidly to invasion of the epithelium or the submucosa by enteric pathogens. Immune cells — including antigen-presenting cells, T cells and granulocytes — secrete cytokines and other factors that modulate the synthesis rate, glycosylation and release of mucins. However, the function of the secreted mucus barrier and its connections with the underlying epithelium and immune cells is often overlooked by those studying mucosal host–pathogen interactions. In this Review, we describe the constituents of the extracellular and cell-associated mucin barrier and provide an overview of the interaction of commensal and pathogenic bacteria with this important defence layer.
The extracellular mucus barrier
Mucus is found throughout the entire gastrointestinal tract but varies in thickness, ranging from 700 μm in the stomach and large intestine to 150–300 μm in the small intestine3 (Fig. 1). The barrier consists of two layers: a thinner inner mucus layer, which is physically difficult to dislodge and is sterile, and a thicker outer mucus layer, which is more easily dispersed and not sterile1,2. The inner layer is much thinner in the small intestine than in the large intestine3, a trait that may be necessary to accommodate the need for nutrient absorption. The extracellular mucus barrier has three major components: secreted mucins, nonspecific antimicrobials and specific antimicrobial immunoglobulins. The major protein components of mucus are listed in Table 1. Mucins also have important functions at the cell surface, as discussed below.
Cells that produce the secreted barrier. The secreted (gel-forming) mucin glycoproteins that form the major macromolecular constituents of mucus are produced by specialized mucus or goblet cells that are found throughout the gastrointestinal tract (Fig. 1). The other major secretory cells within the gastrointestinal tract are the Paneth cells, which are primarily found adjacent to stem cells deep in the crypts of the small intestine (Fig. 1). Paneth cells are identified by their characteristic intracellular granules containing a range of antimicrobial molecules that are secreted into the mucus to ensure sterility of the stem cell niche4. Mucus is continuously cleared from the mucosal surface: in the respiratory tract, mucus is removed by the action of cilia and by coughing, whereas in the gastrointestinal tract, the outer mucus layer is continuously shifted by the peristaltic movement of luminal food and faecal material. Genetic defects in intestinal goblet cells and Paneth cells can lead to spontaneous inflammation and increased susceptibility to infection, and the large protein synthesis load of these secretory cells renders them susceptible to endoplasmic reticulum stress. Defects leading to protein misfolding or an aberrant unfolded-protein response in intestinal secretory cells lead to intestinal inflammation5,6,7,8,9,10,11,12. For example, missense mutations in the mouse mucin 2 gene (Muc2) that result in misfolding of the protein cause goblet cell and Paneth cell stress, as well as spontaneous colonic inflammation5. Similarly, when the gene encoding the transcription factor XBP1 (a key component of the unfolded-protein response) is conditionally deleted in mouse intestinal epithelial cells, Paneth cell and goblet cell stress occurs, leading to spontaneous inflammation in the ileum8. In addition, mice with this conditional deletion are more susceptible to infection with enteric bacterial pathogens8.
Secreted mucin glycoproteins. Secreted mucins are large, heavily O-glycosylated glycoproteins that are assembled into homo-oligomers which give mucus its viscous properties13. There are five oligomerizing secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC19) and one non-oligomerizing secreted mucin (MUC7)13, which are produced in different regions of the gastrointestinal tract (Table 1). The oligomerized mucins form homo-oligomers via inter-molecular disulphide bonds that are formed between the cysteine-rich D-domains found at the amino and carboxyl termini of these mucins (Fig. 2). N-glycosylation and C-terminal dimerization occur in the endoplasmic reticulum, followed by O-glycosylation in the Golgi and N-terminal multimerization of the dimers as mucins are packaged into granules in preparation for secretion13. There is some controversy about whether N-terminal oligomerization forms linear thread-like polymers or whether trimerization or other patterns of oligomerization result in more complex lattice-like structures (discussed in Ref. 13). The granules form the characteristic goblet cell thecae and are secreted, both constitutively and in response to extracellular stimuli, through a highly regulated process that involves the transport of granules via actin remodelling, granule tethering to the membrane and granule exocytosis14. Reduction of mucin disulphide bonds disassembles the oligomers and greatly reduces mucus viscosity, demonstrating the importance of oligomerization. Reduction of mucins is a routine biochemical procedure that is used for the analysis of these molecules, and is also used clinically to reduce mucus viscosity in respiratory diseases, including cystic fibrosis15. In addition to homo-oligomerization, it is possible that covalent or non-covalent crosslinking occurs between mucin molecules and other components of the mucus (discussed below).
a | Mucin 2 (MUC2) is the major component of the secreted mucus barrier in the small and large intestines. The variable number of tandem repeat (VNTR) domains are heavily O-glycosylated, and the amino- and carboxy-terminal D-domains are involved in homo-oligomerization. The structure of MUC1 is typical of the structures of the large number of cell surface mucins in the gastrointestinal tract. The extracellular VNTR domain is heavily O-glycosylated, and the protein is N-glycosylated near the SEA module. The cytoplasmic domain of MUC1 is involved in intracellular signal transduction. b | Cell surface mucins are cleaved into two subunits in the endoplasmic reticulum, inserted into the membrane and N-glycosylated. In the endoplasmic reticulum, secreted mucins are N-glycosylated and dimerize via their C-terminal domains. Both cell surface and secreted mucins are O-glycosylated in the Golgi. Following completion of O-glycosylation, the dimers of the secreted mucins undergo N-terminal oligomerization and are packed into granules for secretion. c | Oligomerized mucins either form a tangled web of linear polymers following N-terminal dimerization, or a complex molecular network following N-terminal trimerization (one possible network is shown). Other non-mucin molecules may form non-covalent interactions with mucins. Following secretion, granules hydrate and swell by as much as 100–1,000-fold as mucus is formed.
The complex array of oligosaccharides on the large, central glycosylated domains of mucins represent multiple potential ligands for microbial adhesins and can be utilized as an energy source by some commensal microorganisms in the outer mucus layer. The identity of these oligosaccharides is determined by the expression pattern of the glycosyltransferases within the Golgi of the goblet cells. Mass spectrometry has revealed that >100 different oligosaccharides are present on human colonic MUC2 (Ref. 16). The expression of glycosyltransferases differs between sites in the gastrointestinal tract and can be modulated by innate and adaptive immunity. Genetic variation in the genes encoding these transferases results in a diversity of mucin carbohydrate structures in human populations; one example is the variation in oligosaccharides of the Lewis blood group antigens. Even the same mucin proteins can be differentially glycosylated by different populations of goblet cells within the same tissue. For example, it has been shown using histochemical staining in the human colon that adjacent goblet cells within individual crypts produce different mucin glycoforms that then form striated layers in the secreted mucus. In addition, there are occasionally individual crypts that contain goblet cells producing a different mucin glycoform to those produced by the goblet cells of surrounding crypts17. In addition to this variation in glycosylation within a species, there is considerable variation between species, and this variation may be an important contributor to the species specificity of both commensal bacteria and pathogens18.
Antimicrobial components of the secreted barrier. The mucus gel provides a matrix for the retention of antimicrobial molecules in the mucosal environment; these molecules are produced throughout the gastrointestinal tract, primarily by Paneth cells, and include α-defensins, cathlecidins, lysozymes, angiogenin 4, secretory phospholipase A2, lipopolysaccharide-binding protein, collectins, histatins, and lectins such as REGIIIα (also known as HIP and PAP) and REGIIIγ4,19,20 (Table 1). Although these molecules have a wide variety of structures, many are microbicidal lectins or small, amphipathic, cationic peptides, and interact with and disrupt microbial cell membranes21,22. Direct interactions with mucins can facilitate the retention of antimicrobial molecules; for example, in saliva MUC7 binds to histatin 1 and statherin23, and MUC5B binds to histatin 1, histatin 3, histatin 5 and statherin24. In addition, mucin glycoproteins can themselves have direct antimicrobial properties that limit the growth of microorganisms in the mucus. The terminal mucin monosaccharide α(1,4)-linked N-acetylglucosamine, found on gastric MUC6, inhibits the synthesis of Helicobacter pylori cell wall components25 and has therefore been suggested to limit H. pylori growth within the gastric mucus. MUC7 has a histatin peptide domain with direct antimicrobial activity against Candida spp. and thus limits the growth of yeast in saliva26. The secretory antibodies, immunoglobulin A (IgA) and IgG, which are produced by B cells in the lamina propria of the intestine and are secreted into the mucus by the epithelial cells, are very important components of the mucosal barrier27. These antibodies influence the commensal microbiota and contribute substantially to the capacity of the mucus to retain and clear potential pathogens; these antibodies also function more efficiently through their retention in the mucus environment than they would if they were simply dispersed into the intestinal lumen27,28. However, although the Paneth cells in the ileum secrete many antimicrobial molecules, there are still around 105–107 bacteria per gram of luminal material in this region of the intestine. This is likely to reflect the fact that the microbicidal molecules in the mucus are at their highest concentration close to the mucosal surface and that therefore the concentration at the luminal side of the mucus layer is sufficiently low to allow the bacteria to survive.
Interestingly, there are no examples of individual antimicrobial molecules for which a deficiency results in either spontaneous inflammation or susceptibility to infection by enteric pathogens, which is likely to reflect the substantial overlap in the expression of antimicrobial molecules. However, there are examples that highlight the important role of specific antimicrobial peptides in defence against infection. Mice lacking matrilysin (MMP7), the protease that processes pro-α-defensins into active compounds, are impaired in intestinal clearance of Escherichia coli and have more severe systemic infection with Salmonella enterica subsp. enterica serovar Typhimurium following oral inoculation than wild-type mice29. In addition, mice that express human defensin 5 have increased resistance to S. Typhimurium infection30. When interpreting these data, it is important to recognize that an altered production of antimicrobial molecules by Paneth cells results in changes to the commensal microbiota31, which in turn could change the niche for, and host susceptibility to, individual enteric pathogens and chronic inflammation32.
Proteomic studies have shown that mucus contains a large number of proteins in addition to mucins33. For many of these molecules, their function within the mucus remains unclear, although many may interact with the mucin glycoproteins, as has been shown for IgG-Fc-binding protein (FcGBP)33, which has cysteine-rich domains that are homologous to MUC2 in addition to multiple domains capable of binding IgG. These additional proteins may influence the rheological and/or functional properties of mucus.
Biophysical properties of the secreted barrier. The highly hydrated, viscous mucus gel is necessarily porous to allow the diffusion of macromolecules that are required for gastrointestinal secretion and absorption (most important in the small intestine), but it nonetheless provides an effective biophysical barrier to particulate matter, including microorganisms. Virus-sized particles (<500 nm) can readily diffuse through undiluted cervical mucus, whereas mucus is an impermeable elastic barrier to bacterium-sized particles34,35. However, when small particles, including viruses, interact with components of the mucus, the particles can be trapped, mimicking what happens when viruses bind the mucins themselves or to antibodies within the mucus35.
Goblet cells secrete several other proteins into the mucus in addition to mucins, such as trefoil peptides. Trefoil peptides have been implicated in non-covalent binding to mucins36,37,38 and appear to be capable of altering the biophysical and protective properties of the mucus39,40. Some individual trefoil peptides are tightly co-expressed with specific secreted gel-forming mucins. When two or more different mucins are mixed in the mucus, this is likely to alter its rheological properties. The ionic environment can also influence the biophysical properties of the mucus; for example, Ca2+ concentrations change the viscosity of human saliva and the ability of particles to diffuse through it41, and bicarbonate () has also been shown to influence mucus viscosity42.
The cell surface mucin barrier
Although the rheological and biochemical properties of the extracellular mucus layer present a considerable challenge to enteric microorganisms, pathogens can sometimes breach this barrier and reach the cell surface. Cell surface mucins have an important role in the response to microorganisms that reach the cell surface.
Cell surface mucins in the glycocalyx. The apical surface of mucosal epithelial cells is usually covered by dense microvilli and a complex glycocalyx containing high levels of cell surface transmembrane mucin glycoproteins. Cell surface mucins produced in the gastrointestinal tract include MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16 and MUC17 (Ref. 43) (Table 1). Although the relative expression of these mucins varies between different regions of the tract, and between different cell types within each region, many gastrointestinal cells express multiple members of this cell surface mucin family. All of these mucins contain an extracellular O-glycosylated domain, which is often extremely large and is likely to form a long filamentous structure that is predicted to be larger than the microvilli themselves in some cases43. These large mucins carry oligosaccharides that mimic other sugars found in the glycocalyx and may be ligands for microbial adhesins. For example, several of the Lewis blood group carbohydrate structures are also found on glycosphingolipids that are present in the intestinal apical glycocalyx44. The cell surface mucins are cleaved during biosynthesis and reach the cell surface as two non-covalently linked subunits that are joined at the SEA module, allowing subsequent release of the glycosylated extracellular domain from the surface45,46. In addition to constitutive shedding of the mucin domain owing to disassociation of the subunits at the SEA module, shedding is enhanced following binding by bacteria47 and can be promoted by extracellular proteases48,49.
Cell surface mucins in protection from mucosal infection. Cell surface mucins are required to protect against enteric pathogens that penetrate the inner mucus layer. These mucins are likely to be the first molecules that invading microorganisms interact with at the cell surface and thus can limit pathogen binding to other glycoproteins and neutralize the pathogen. Many enteric pathogens have secretion systems for the introduction of microbial proteins into intestinal epithelial cells in order to disturb epithelial physiology to their advantage. Binding of the bacteria to the cell surface is essential for activation of these secretory systems. Consequently, many pathogens have evolved adhesins for the ligands that are found on the apical surface of intestinal epithelial cells. Emerging evidence supports an important role for the cell surface mucins in limiting the pathology that results from bacterial infections of the gastrointestinal tract. Despite some redundancy in the expression of cell surface mucins, mice deficient in MUC1 are more susceptible to infection with the gastric pathogen H. pylori50,51 and the gastrointestinal pathogen Campylobacter jejuni52. Compared with wild-type mice, Muc1−/− mice develop a fivefold-higher density of infection as early as 1 day after exposure to H. pylori50, demonstrating that this mucin limits the density of H. pylori, the majority of which is found deep in the gastric mucus53. Furthermore, Muc1−/− mice develop much more severe chronic inflammation, showing that cell surface mucins can limit the pathology resulting from chronic mucosal infection50,51. Similarly, in acute infection with C. jejuni, Muc1−/− mice rapidly develop systemic infection, demonstrating that MUC1 effectively limits translocation of this enteric pathogen52. Using in vitro co-cultures of H. pylori and gastric epithelial cells, we have shown that MUC1 is shed from the cell surface in response to infection and thus acts as a decoy to limit adhesion of the bacteria to the cell surface47 (Fig. 3). Interestingly, this blocking effect of MUC1 is influenced by the binding of bacteria to the mucin, but works even when bacteria lack adhesins for the mucin carbohydrates. H. pylori can express two main adhesins for mucin carbohydrates, sialic acid-binding adhesin (SabA) and blood group antigen-binding adhesin (BabA), which vary in their expression levels in infected human populations and affect pathogenicity54,55. When H. pylori expresses SabA and BabA, the mucin binds the bacterium and is shed from the cell surface. When H. pylori lacks the adhesins, the mucin is not shed but still effectively blocks adhesion of the bacterium to the cell surface, presumably by steric hindrance47 (Fig. 3). It should be stressed that cell surface mucins are likely to exist in complex molecular networks in the glycocalyx, and this model is therefore probably an oversimplification of the molecular events that occur during bacterial attachment to mucins.
a | Contact of an enteric pathogen with the cell surface stimulates secretion of stored mucus from goblet cells. Mucin granules expand (increasing in size by 100–1,000-fold) on hydration and surround pathogens with fresh mucus containing antimicrobial molecules. b | Engagement of cell surface mucins by pathogens initiates signalling through the cytoplasmic domain of these cell signalling proteins. c | If a pathogen binds to a cell surface mucin, the mucin extracellular domain is shed, releasing the pathogen from the cell surface. d | Enteric pathogens that do not bind to the cell surface mucins are excluded by steric hindrance.
Polymorphisms in MUC1 have been associated with the development of gastritis and gastric cancers following H. pylori infection in humans56,57,58, providing further evidence of the importance of this mucin in chronic H. pylori infection. Furthermore, secretor status is determined by polymorphic inheritance of galactoside 2-α-L-fucosyltransferase 2 (FUT2), an enzyme that influences glycosylation of both cell surface and secreted mucins (specifically of oligosaccharide ligands for H. pylori adhesins) and influences the distributions of specific adhesin-carrying strains of H. pylori in human populations55. Mice lacking FUT2 show altered glycosylation of mucins and altered binding of H. pylori to the gastric mucosa59. In rhesus monkeys infected with H. pylori, time-dependent changes occur in glycosylation that influence the host–pathogen interaction and are dependent on secretor status60,61, further underlining the significance of the oligosaccharide ligands for this bacterial species. In another example of the importance of Lewis blood group antigens, human noroviruses, which can cause severe gastroenteritis, bind to the cell surface via Lewis epitopes, but individuals that do not secrete these antigens are protected from norovirus infections62.
In addition to impeding bacterial adhesion to the cell surface, there is emerging evidence that the cell surface mucins modulate epithelial cell inflammatory signalling through their cytoplasmic domains63. MUC1 signalling, which is probably activated by interaction of the extracellular domain with bacteria and/or by shedding of the extracellular domain, can modulate activation of the nuclear factor-κB (NF-κB) family of transcription factors, which are the master regulators of inflammatory signalling51,64. Although the consequences of signalling by most of the remaining members of the cell surface mucin family remain to be elucidated, it is clear that the cell surface mucins are important elements of the interactions between enteric pathogens and mucosal epithelial cells, and important modulators of the subsequent immune responses. Interestingly, MUC1 polymorphisms have been recently linked with Crohn's disease, which is a chronic intestinal inflammatory condition for which both pathogens and innate immunity defects have been implicated as potential aetiological contributors65.
Pathogens and the mucosal barrier
Gastrointestinal epithelial cells are tightly linked via intracellular junctions that form a contiguous barrier which is resistant to microbial passage. Thus, there are three levels to the barrier to infection: secreted mucus, the apical glycocalyx and epithelial tight junctions. However, enteric pathogens have evolved a wide range of specific strategies to either penetrate or circumvent the secreted and cellular barriers to infection. The importance of the mucus barrier is underlined by recent reports that mice deficient in MUC2 develop severe, life-threatening disease when infected with the attaching and effacing E. coli-like pathogen Citrobacter rodentium66, and show delayed clearance of the nematode parasite Trichuris muris67. These mice also develop spontaneous intestinal inflammation68, consistent with the previously demonstrated non-physiological exposure of the commensal microbiota to the epithelium when mucus is depleted1. Not surprisingly, pathogens have evolved many ways of evading the mucosal barrier. These include mechanisms to allow efficient penetration of the mucus, enzymes that degrade the mucus, pathways that allow evasion of the barrier, and disruption of the cells that produce the barrier (Fig. 4).
a | Pathogens can penetrate the mucus barrier physically, through flagella-mediated motility or through enzymatic degradation of the mucus. This can result in both pathogens and commensal bacteria reaching the epithelium. b | Microfold (M) cells in the small intestine sample the commensal microbiota to control the specificity of non-inflammatory, secretory immunoglobulin A-dominated immune responses to the normal microbiota. The M cells are found in the dome epithelium, which is not covered by a thick mucus layer; thus, pathogens can avoid the mucus barrier by entering via the M cells. c | Many pathogens secrete toxins that can diffuse through the mucus. These can disrupt the tight junctions between the epithelial cells, block epithelial cell growth and disrupt mucus production. The reduction in mucus levels will allow pathogens to reach the cell surface.
Penetration and disruption of the mucus barrier. Many enteric pathogens are chemoattracted to mucus and use it as a cue to upregulate genes involved in pathogenicity69,70. For example, after in vitro exposure to purified human MUC2, C. jejuni upregulated genes encoding cytolethal distending toxin, vacuolating cytotoxin, surface-exposed lipoprotein (JlpA), Campylobacter invasion antigen B, the multidrug efflux system, putative mucin-degrading enzymes, flagellin A and putative rod shape-determining proteins70. The mucus environment can also be advantageous for the pathogen; for example, H. pylori is protected from the low acidity in the stomach by residing deep in the mucus layer53.
One common feature of enteric pathogens is the presence of flagella, which allow the bacteria to propel themselves within the viscous mucus environment. Pathogens with disturbed flagellar function have reduced pathogenicity, underlining the importance of motility in disease (it should be recognized that flagella are also involved in adhesion and activation of immune responses)71,72. Furthermore, some pathogens can alter the mucus in their microenvironment to increase their motility, as exemplified by H. pylori, which can increase the pH of its local environment, thus decreasing the viscoelasticity of the surrounding mucus73. Despite pathogen motility, mucus can aggregate and remove microorganisms; consequently, many microorganisms have evolved enzymes to degrade the mucus. These enzymes include glycosidases, which degrade the mucin oligosaccharides, exposing the mucin peptide backbone to proteases while also removing decoy carbohydrates for microbial adhesins (Table 2). Proteolytic cleavage of mucins causes disassembly of the oligomerized mucin macromolecules, resulting in substantially diminished mucus viscosity, dispersal of the mucus, and diffusion and dilution of antimicrobial molecules. The protozoan parasite Entamoeba histolytica cleaves MUC2 in the non-glycosylated oligomerization domains, thus breaking down the macromolecular structure and reducing mucus viscosity74. An example of both a response to mucus and the degradation of mucus comes from Vibrio cholerae, which switches on hapA after exposure to intestinal mucus; this gene encodes haemagglutinin protease (Hap), which has mucolytic activity that is required for translocation through mucin-containing gels75.
Mucus degradation is not limited to pathogens, as some commensal intestinal bacteria are also mucolytic, and can use mucin glycoproteins as an energy source76,77,78,79 and also to provide substrates for other non-pathogenic bacteria in the outer mucus layer79. In contrast to the pathogens, these bacteria are typically strictly anaerobic and do not penetrate the inner mucus layer. Complex populations of commensal mucolytic bacteria dominate the niche in the outer mucus layer in vivo, and this may favour the host by excluding pathogens. When anaerobic mucolytic bacteria are combined in vitro, there is more complete degradation of MUC2 and more bacterial growth than when these bacteria are found in isolation79, explaining the importance of complex populations of commensal microorganisms, and their ability to exclude pathogens79. The distribution of commensal mucolytic bacteria varies between members of the population and is probably determined in part by polymorphic host glycosylation18.
Furthermore, depending on nutrient availability, microorganisms are able to switch from utilizing dietary fibre to using mucin glycoproteins as their major source of energy, linking the host diet with mucin degradation and, thus, with the functional efficacy of the mucus barrier78. Mucus production requires substantial energy, and mucin glycoproteins are rich in the essential amino acid threonine. Therefore, low-protein diets can result in decreased mucin biosynthesis and a diminished mucus layer80. This could affect the frequency and severity of enteric infections, especially in the developing world.
There are no empirical data on how microbial mucin-degrading enzymes affect the function of the cell surface mucins, although it is likely that these enzymes would also impair the cell surface mucin-mediated block of microbial adhesion to the cell surface, and would therefore promote pathogenicity.
Avoidance of the mucus barrier. Rather than penetrate the mucus directly, a large number of enteric pathogens have evolved strategies to infect the host via the normal physiological sampling of bacteria that is carried out by the mucosal immune system in the small intestine. Microfold (M) cells capture and present microorganisms that are representative of the commensal microbiota to underlying antigen-presenting cells81,82. This allows the initiation of non-inflammatory, secretory IgA-dominated immune responses against the normal microbial microorganisms in the lumen of the gut. This highly controlled immune response is central to maintaining the function of the mucosal barrier and intestinal homoeostasis83,84,85. M cells reside in the dome epithelium, which overlays the Peyer's patches of the small intestine. The dome epithelium lacks goblet cells and therefore is not covered by a thick mucus layer, leaving holes in the mucus barrier that allow the M cells to sample the gut microbiota. M cells can be identified by their reduced density of microvilli and by alterations in the oligosaccharides in their glycocalyx, suggesting that they also have an altered expression of cell surface mucins86,87. The altered cell surface of these cells and their ability to transcytose commensal microorganisms makes them inherently susceptible to pathogens. Many microaerophilic or aerobic pathogens specifically enter the intestinal mucosa via the M cells, including bacteria (such as S. Typhimurium, Shigella flexneri, Yersinia enterocolitica and V. cholerae), viruses (such as reoviruses, HIV-1 and poliovirus) and parasites (such as Cryptosporidium spp.)81,88,89. As the dome epithelium is restricted to the small intestine, pathogens in the large intestine need to penetrate the mucus barrier to reach the cell surface. Upon reaching the epithelial surface, many pathogens spread laterally by disrupting the epithelium and binding to the vulnerable basolateral surface of epithelial cells.
Disruption of epithelial integrity. Many pathogens secrete toxins that can disrupt epithelial cells and consequently alter the production of mucins and antimicrobial molecules. These toxins can result in lysis, induction of apoptosis, growth inhibition, cell cycle arrest, disruption of intracellular tight junctions and modulation of inflammatory signalling. Such damage not only facilitates further invasion by the pathogen but also can result in the normal commensal microorganisms gaining access to the epithelium and submucosa. Infection and inflammation in the intestine alters the commensal microbiota in response to the changed mucosal microenvironment90,91,92,93. Similarly, non-infectious damage to the gastrointestinal epithelium can change the microbial populations94 and facilitate enteric infections by compromising mucosal barrier function.
After pathogens have reached the epithelium, many of them disrupt the tight junctions between adjacent epithelial cells; this exposes the vulnerable lateral membranes that are not protected by the glycocalyx, allowing the pathogen to access underlying tissues and promoting diarrhoea (reviewed in Ref. 95). Examples of gastrointestinal microorganisms which interfere with cell–cell adhesion include S. flexneri96, Listeria monocytogenes97, enteropathogenic E. coli98, Porphyromonas gingivalis99, H. pylori100 and rotaviruses101. Electron microscopy studies in isolated porcine ileal loops show that S. Typhimurium preferentially targets sites where infected epithelial cells are being extruded from the epithelium102. In addition to their blocking role on the cell surface, cell surface mucins seem to modulate epithelial cell responses to toxin-induced damage. For example, MUC1 protects adenocarcinoma cells from apoptosis after exposure to DNA-damaging drugs in vitro103,104; this probably occurs because of the known role of MUC1 in protecting mucosal epithelial cells from pathogen toxins such as the C. jejuni cytolethal distending toxin52.
Mucin dynamics
The mucosal barrier should not be viewed as a static barrier, as its constituents and their release are modulated by the luminal microenvironment and by neural, endocrine and immune factors. Although mucins are constitutively secreted, infection of mucosal surfaces can result in a rapid release of stored mucin granules to bolster the barrier and exclude pathogens14. A range of environmental stimuli can alter the rate of mucin release, including inflammatory cytokines, prostaglandins, cholinergic stimuli, lipopolysaccharide, bile salts, nucleotides, nitric oxide, vasoactive intestinal peptides and neutrophil elastase105,106,107,108,109,110,111,112,113,114,115,116. The release of granules containing stored secreted mucins from goblet cells can happen rapidly through compound exocytosis and results in a 100–1,000-fold expansion in mucin volume upon hydration117,118. Thus, mucosal tissues can rapidly replace mucus that may have been removed mechanically or by degradation by pathogens. Shedding of cell surface mucins is induced by bacterial attachment, possibly in response to shear forces that result from binding to motile bacteria, and can also be modulated by the activity of proteases that are released at the apical cell surface.
Steady-state regulation of mucin expression. The major components of the mucosal barrier are expressed constitutively, as continuous mucus production and secretion are required to replace the mucus that is degraded by luminal bacteria and that which is lost with the movement of luminal contents. Dynamic changes to the mucosal barrier in response to local factors are summarized in Fig. 5. The production rates of both cell surface and secreted mucins, as well as their glycosylation, are modulated by the normal microbiota and can be dynamically regulated by both innate and adaptive immunity following infection. Similarly, the production rates of antimicrobial molecules are regulated by both the normal microbiota and inflammatory signals. In germ-free conditions, there are fewer intestinal goblet cells and reduced intracellular storage of mucin granules in the thecae119,120,121,122, as well as reduced expression of some Paneth cell molecules, such as angiogenin 4 and REGIIIγ123,124. The microbiota in the gut also influences mucin glycosylation, as re-establishment of a commensal microbiota in germ-free animals alters mucin glycosylation120,125,126,127. However, these studies have used only crude analyses of mucin carbohydrates, and detailed analyses of individual mucins are still required to detail the specific changes in glycosylation.
a | The constitutive release of mucins and antimicrobial molecules is influenced by the microbial flora. Post-infection, the secretion of mucus and antimicrobial molecules increases and pathogen-specific immunoglobulins are also secreted. b | The recognition of pathogens leads to the production of host inflammatory factors that, in turn, induce the differentiation of secretory cells and alterations in mucus composition through altered transcription of mucins and the glycosyltransferases that act on them. The recognition of pathogen-associated molecular patterns (PAMPs) activates Toll-like receptor (TLR) and NOD-like receptor (NLR) signalling pathways, which leads to alterations in the expression of mucin and antimicrobial genes. IL, interleukin; TH, T helper cell.
Regulation by innate and adaptive immunity. In addition to direct regulation of epithelial cells in response to microbial signals, both innate and adaptive immunity can regulate the differentiation of goblet cells, the glycosylation of mucins and the production rates of antimicrobial molecules and of secreted and cell surface mucins in response to infection. The most potent effects are induced by T cell cytokines: interleukin-4 (IL-4) and IL-13, the cytokines that are produced by T helper 2 (TH2) cells in response to parasitic infections, promote goblet cell hyperplasia and substantial increases in mucus production in the intestine128,129. These cytokines induce expression of the transcription factor SPDEF, which drives goblet cell differentiation and the expression of genes involved in mucus biosynthesis and secretion130,131,132,133. In addition, interferon-γ (IFNγ) and IL-17, which are classically produced by TH1 cells and TH17 cells in response to intracellular and extracellular pathogens, respectively, affect goblet cells by increasing mucin production134,135,136,137. In fact, the expression of mucins by mucosal epithelial cells in vitro can be directly upregulated by a broad array of inflammatory cytokines, such as IL-1β, IL-4, IL-6, IL-9, IL-13, IFNs, tumour necrosis factor, nitric oxide and granulocyte proteases108,111,128,138,139,140,141,142,143,144. T cell cytokines also mediate changes in mucin glycosylation, probably representing an attempt by the host to alter the pattern of glycosylation that was unable to prevent infection by the pathogen or parasite145,146,147,148,149,150. Comprehensive studies of the dynamic changes in mucin glycosylation during infection and inflammation are warranted, and should take advantage of modern mass spectrometry-based technologies to rapidly analyse complex mucin oligosaccharides.
Gastrointestinal epithelial cells can directly respond to pathogen-associated molecular patterns (PAMPs) via Toll-like receptor (TLR) and NOD-like receptor (NLR) signalling. However, in intact polarized epithelial layers, the limited expression of TLRs on the apical surface ensures that the cells do not overreact to the high concentration of PAMPs in the intestinal mucosal environment151. The exposure of cultured mucosal epithelial cells to microbial products generally results in an increased production of cell surface and secreted mucins108,152,153,154,155. The L. monocytogenes toxin listeriolysin O not only induces increased biosynthesis of cell surface and secreted mucins, but also rapidly triggers the release of mucins by cultured intestinal cells, and these mucins then inhibit pathogen entry into the cells through a mechanism that is dependent on sialylation of the mucins156. By contrast, there are examples of PAMPs that cause reduced mucin production, which is likely to facilitate infection; for example, the exposure of epithelial cells to H. pylori lipopolysaccharide results in decreased synthesis of gastric mucin157.
Changes in mucins during infection. The control of mucin abundance during enteric infection is complex and depends on patterns of gene expression, on rates of mucin biosynthesis, secretion and shedding, and on rates of microbial degradation in the extracellular environment. Unfortunately, there is a lack of comprehensive studies of these factors for even the most common enteric pathogens. Given the changes in the rates of mucin synthesis, glycosylation, secretion and shedding following infection, it is probable that there are important changes in the biophysical properties of the mucus subsequent to infection, although these changes are also yet to be evaluated. In C. rodentium infection in mice, there was a progressive depletion of most of the intestinal secreted and cell surface mucins that were localized to the site of infection, consistent with increased mucin shedding and secretion in an attempt to eliminate infection66,158. Interestingly, despite this progressive depletion of most mucins, there was a progressive increase in the amount of the cell surface mucin MUC1. During rotavirus infection in mice, MUC2 production is increased, mucin glycosylation changes and mucins isolated from the infected mice effectively neutralize the virus in vitro, consistent with an important role for MUC2 in limiting infections with rotaviruses159. Many enteric parasites trigger changes in mucin production and glycosylation that appear to be associated with successful expulsion of the parasites; this is seen, for example, for T. muris and Gymnophalloides seoi in mice160,161, Teladorsagia circumcincta in sheep162 and Cooperia oncophora in cattle163. In a primate model of H. pylori infection, it was shown that there are changes in mucin glycosylation that affect the ability of H. pylori to bind to the mucins and to the cell surface60,61. These changes differ between acute and chronic infection, presumably owing to alterations in inflammatory factors as the immune response develops. Further research is required to understand the functional significance of mucin alterations during infections.
Conclusion
Microbial enteric infections still represent a major health challenge in the developed world and remain some of the most significant causes of morbidity and mortality in the developing world164. Owing to the nature and site of these infections, and the characteristics of the specific causative pathogens, effective vaccines are yet to be developed for the majority of these infections. Our understanding of the interactions between the pathogens that cause enteric infections, the normal microbial populations in the gut, the secreted mucus barrier and the host mucosal surface is increasing but remains incomplete. Unfortunately, mucosal barrier research is restricted by poor in vitro co-culture systems and by technical difficulties in assessing the rapid and dynamic interactions between pathogens and the mucosal barrier in vivo. Almost all co-culture systems used by researchers in the field use malignant epithelial cells which lack goblet cells and do not form a protective mucus layer. Furthermore, these cells often have a different cell surface mucin expression pattern to that found in vivo, and altered mucin glycosylation165. The field would be dramatically enhanced by systems that would allow the growth of normal differentiated gastrointestinal epithelial cells in culture and by the development of in vivo imaging techniques to permit observation of dynamic events as enteric pathogens interact with undisturbed mucus layers in physiological conditions. Ascertaining the nature of the interactions between the secreted and cell surface mucosal barrier and pathogens should be a priority for future research, as it could lead to novel strategies to bolster the innate defence and to antagonize pathogen strategies to disrupt barrier function.
References
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008). Demonstration of the spatial relationship between the intestinal epithelium, the inner and outer mucus layers and the microbiota.
Johansson, M. E., Holmen Larsson, J. M. & Hansson, G. C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl Acad. Sci. USA 25 Jun 2010 (doi:10.1073/pnas.1006451107).
Atuma, C., Strugala, V., Allen, A. & Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G922–G929 (2001). In situ measurement of the mucus thickness throughout the rodent gastrointestinal tract and observations on the replacement of mucus following its physical removal.
Stappenbeck, T. S. Paneth cell development, differentiation, and function: new molecular cues. Gastroenterology 137, 30–33 (2009).
Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Park, S. W. et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl Acad. Sci. USA 106, 6950–6955 (2009).
Zhao, F. et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev. Biol. 338, 270–279 (2009).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Brandl, K. et al. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc. Natl Acad. Sci. USA 106, 3300–3305 (2009).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Cadwell, K. et al. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145 (2010).
McGuckin, M. A., Eri, R. D., Das, I., Lourie, R. & Florin, T. H. ER stress and the unfolded protein response in intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G820–G832 (2010).
Thornton, D. J., Rousseau, K. & McGuckin, M. A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70, 459–486 (2008).
Davis, C. W. & Dickey, B. F. Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol. 70, 487–512 (2008).
Rogers, D. F. Mucoactive agents for airway mucus hypersecretory diseases. Respir. Care 52, 1176–1193; discussion 1193–1177 (2007).
Larsson, J. M., Karlsson, H., Sjovall, H. & Hansson, G. C. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology 19, 756–766 (2009).
Matsuo, K., Ota, H., Akamatsu, T., Sugiyama, A. & Katsuyama, T. Histochemistry of the surface mucous gel layer of the human colon. Gut 40, 782–789 (1997).
Henrissat, B., Surolia, A. & Stanley, P. in Essentials of Glycobiology. 2nd edn (eds Varki, A. et al.) (Cold Spring Harbor Laboratory Press, New York, 2009).
Ouellette, A. J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 26, 547–553 (2010).
Porter, E. M., Bevins, C. L., Ghosh, D. & Ganz, T. The multifaceted Paneth cell. Cell. Mol. Life Sci. 59, 156–170 (2002).
White, S. H., Wimley, W. C. & Selsted, M. E. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 5, 521–527 (1995).
Hristova, K., Selsted, M. E. & White, S. H. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J. Biol. Chem. 272, 24224–24233 (1997).
Bruno, L. S. et al. Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim. Biophys. Acta 1746, 65–72 (2005).
Iontcheva, I., Oppenheim, F. G. & Troxler, R. F. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline rich proteins, statherin, and histatins. J. Dent. Res. 76, 734–743 (1997).
Kawakubo, M. et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305, 1003–1006 (2004). Evidence that a mucin carbohydrate can directly inhibit the growth of a mucus-residing pathogen.
Gururaja, T. L. et al. Candidacidal activity prompted by N-terminus histatin-like domain of human salivary mucin (MUC7). Biochim. Biophys. Acta 1431, 107–119 (1999).
Strugnell, R. A. & Wijburg, O. L. The role of secretory antibodies in infection immunity. Nature Rev. Microbiol. 8, 656–667 (2010).
Phalipon, A. et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115 (2002).
Wilson, C. L. et al. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286, 113–117 (1999).
Salzman, N. H., Ghosh, D., Huttner, K. M., Paterson, Y. & Bevins, C. L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422, 522–526 (2003). In vivo evidence that human intestinal defensins can protect against enteric pathogens.
Salzman, N. H. et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nature Immunol. 11, 76–83 (2010).
Salzman, N. H. Microbiota–immune system interaction: an uneasy alliance. Curr. Opin. Microbiol. 14, 99–105 (2011).
Johansson, M. E., Thomsson, K. A. & Hansson, G. C. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J. Proteome Res. 8, 3549–3557 (2009).
Lai, S. K., Wang, Y. Y., Cone, R., Wirtz, D. & Hanes, J. Altering mucus rheology to “solidify” human mucus at the nanoscale. PLoS ONE 4, e4294 (2009).
Lai, S. K. et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl Acad. Sci. USA 104, 1482–1487 (2007).
Tomasetto, C. et al. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology 118, 70–80 (2000).
Newton, J. L., Allen, A., Westley, B. R. & May, F. E. B. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut 46, 312–320 (2000).
Ruchaud-Sparagano, M. H., Westley, B. R. & May, F. E. The trefoil protein TFF1 is bound to MUC5AC in human gastric mucosa. Cell. Mol. Life Sci. 61, 1946–1954 (2004).
Kindon, H., Pothoulakis, C., Thim, L., Lynchdevaney, G. & Podolsky, D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology 109, 516–523 (1995).
Thim, L., Madsen, F. & Poulsen, S. S. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Invest. 32, 519–527 (2002).
Raynal, B. D., Hardingham, T. E., Sheehan, J. K. & Thornton, D. J. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J. Biol. Chem. 278, 28703–28710 (2003).
Garcia, M. A., Yang, N. & Quinton, P. M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J. Clin. Invest. 119, 2613–2622 (2009).
Hattrup, C. L. & Gendler, S. J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, 431–457 (2008).
Henry, S. et al. Structural and immunochemical identification of Le(a), Le(b), H type 1, and related glycolipids in small intestinal mucosa of a group O Le(a-b-) nonsecretor. Glycoconj. J. 14, 209–223 (1997).
Wreschner, D. H. et al. Generation of ligand-receptor alliances by “SEA” module-mediated cleavage of membrane-associated mucin proteins. Protein Sci. 11, 698–706 (2002).
Macao, B., Johansson, D. G., Hansson, G. C. & Hard, T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nature Struct. Mol. Biol. 13, 71–76 (2006).
Linden, S. K. et al. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 5, e1000617 (2009). Elucidation of the mechanisms by which cell surface mucins limit pathogen adhesion to the cell surface.
Thathiah, A., Blobel, C. P. & Carson, D. D. Tumor necrosis factor-α converting enzyme/ADAM 17 mediates MUC1 shedding. J. Biol. Chem. 278, 3386–3394 (2003).
Thathiah, A. & Carson, D. D. MT1-MMP mediates MUC1 shedding independently of TACE/ADAM17. Biochem. J. 382, 363–373 (2004).
McGuckin, M. A. et al. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133, 1210–1218 (2007). First demonstration that the deficiency of a cell surface mucin predisposes the host to more severe chronic inflammation during a chronic infection.
Guang, W. et al. Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen. J. Biol. Chem. 285, 20547–20557 (2010).
McAuley, J. L. et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313–2324 (2007). First demonstration that a cell surface mucin limits both the translocation of enteric pathogens and damage to the gut.
Schreiber, S. et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc. Natl Acad. Sci. USA 101, 5024–5029 (2004).
Mahdavi, J. et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297, 573–578 (2002).
Aspholm-Hurtig, M. et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305, 519–522 (2004).
Carvalho, F. et al. MUC1 gene polymorphism and gastric cancer–an epidemiological study. Glycoconj. J. 14, 107–111 (1997).
Vinall, L. E. et al. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology 123, 41–49 (2002).
Silva, F. et al. MUC1 polymorphism confers increased risk for intestinal metaplasia in a Colombian population with chronic gastritis. Eur. J. Hum. Genet. 11, 380–384 (2003).
Magalhaes, A. et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology 19, 1525–1536 (2009).
Linden, S. et al. Role of ABO secretor status in mucosal innate immunity and H. pylori infection. PLoS Pathog. 4, e2 (2008).
Cooke, C. L. et al. Modification of gastric mucin oligosaccharide expression in rhesus macaques after infection with Helicobacter pylori. Gastroenterology 137, 1061–1071 (2009).
Lindesmith, L. et al. Human susceptibility and resistance to Norwalk virus infection. Nature Med. 9, 548–553 (2003).
Ueno, K. et al. MUC1 mucin is a negative regulator of Toll-like receptor signaling. Am. J. Respir. Cell. Mol. Biol. 38, 263–268 (2007).
Ahmad, R. et al. MUC1 oncoprotein activates the IκB kinase β complex and constitutive NF-κB signalling. Nature Cell Biol. 9, 1419–1427 (2007). Evidence that signalling by cell surface mucins modulates inflammatory signalling in epithelial cells.
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nature Genet. 42, 1118–1125 (2010).
Bergstrom, K. S. et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6, e1000902 (2010). Finding that there is more severe pathology during bacterial infection in the intestine in the absence of the secreted mucin MUC2.
Hasnain, S. Z. et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138, 1763–1771 (2010). First evidence that secreted mucins are important components of the T H 2-type immune response that mediates expulsion of nematode parasites.
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop Colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006). Finding that a deficiency in intestinal secreted mucins leads to spontaneous intestinal inflammation.
Hugdahl, M. B., Beery, J. T. & Doyle, M. P. Chemotactic behavior of Campylobacter jejuni. Infect. Immun. 56, 1560–1566 (1988).
Tu, Q. V., McGuckin, M. A. & Mendz, G. L. Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. J. Med. Microbiol. 57, 795–802 (2008).
Ottemann, K. M. & Lowenthal, A. C. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 70, 1984–1990 (2002).
Ramos, H. C., Rumbo, M. & Sirard, J. C. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12, 509–517 (2004).
Celli, J. P. et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl Acad. Sci. USA 106, 14321–14326 (2009). Demonstration that H. pylori reduces mucus visocosity in its microenvironment to promote bacterial motility.
Lidell, M. E., Moncada, D. M., Chadee, K. & Hansson, G. C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl Acad. Sci. USA 103, 9298–9303 (2006). Finding that there is specific enzymatic destruction of MUC2 polymers in mucus by an enteric amoebic parasite.
Silva, A. J., Pham, K. & Benitez, J. A. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149, 1883–1891 (2003).
Deplancke, B. et al. Selective growth of mucolytic bacteria including Clostridium perfringens in a neonatal piglet model of total parenteral nutrition. Am. J. Clin. Nutr. 76, 1117–1125 (2002).
Sonnenburg, J. L., Angenent, L. T. & Gordon, J. I. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nature Immunol. 5, 569–573 (2004).
Sonnenburg, J. L. et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959 (2005). Exploration of the altered utilization of mucin carbohydrates by an individual commensal bacterium under different host dietary conditions.
Png, C. W. et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 105, 2420–2428 (2010).
Law, G. K., Bertolo, R. F., Adjiri-Awere, A., Pencharz, P. B. & Ball, R. O. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1293–G1301 (2007).
Siebers, A. & Finlay, B. B. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 4, 22–29 (1996).
Neutra, M. R., Mantis, N. J., Frey, A. & Giannasca, P. J. The composition and function of M cell apical membranes: implications for microbial pathogenesis. Semin. Immunol. 11, 171–181 (1999).
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
Macpherson, A. J., McCoy, K. D., Johansen, F. E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Endt, K. et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 6, e1001097 (2010).
Lelouard, H., Reggio, H., Mangeat, P., Neutra, M. & Montcourrier, P. Mucin-related epitopes distinguish M cells and enterocytes in rabbit appendix and Peyer's patches. Infect. Immun. 67, 357–367 (1999).
Lelouard, H. et al. Glycocalyx on rabbit intestinal M cells displays carbohydrate epitopes from Muc2. Infect. Immun. 69, 1061–1071 (2001).
Vazquez-Torres, A. & Fang, F. C. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 3, 54–59 (2000).
Jones, B., Pascopella, L. & Falkow, S. Entry of microbes into the host: using M cells to break the mucosal barrier. Curr. Opin. Immunol. 7, 474–478 (1995).
Walk, S. T., Blum, A. M., Ewing, S. A., Weinstock, J. V. & Young, V. B. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm. Bowel Dis. 16, 1841–1849 (2010).
Frank, D. N. et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 189–194 (2010).
Willing, B. P. et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139, 1844–1854 (2010).
Hoffmann, C. et al. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect. Immun. 77, 4668–4678 (2009).
Dalby, A. B., Frank, D. N., St. Amand, A. L., Bendele, A. M. & Pace, N. R. Culture-independent analysis of indomethacin-induced alterations in the rat gastrointestinal microbiota. Appl. Environ. Microbiol. 72, 6707–6715 (2006).
Guttman, J. A. & Finlay, B. B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788, 832–841 (2009).
Sakaguchi, T., Kohler, H., Gu, X., McCormick, B. A. & Reinecker, H. C. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell. Microbiol. 4, 367–381 (2002).
Mengaud, J., Ohayon, H., Gounon, P., Mege, R. M. & Cossart, P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84, 923–932 (1996).
Goosney, D. L., Gruenheid, S. & Finlay, B. B. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 16, 173–189 (2000).
Katz, J., Sambandam, V., Wu, J. H., Michalek, S. M. & Balkovetz, D. F. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect. Immun. 68, 1441–1449 (2000).
Amieva, M. R. et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, 1430–1434 (2003).
Nava, P., Lopez, S., Arias, C. F., Islas, S. & Gonzalez-Mariscal, L. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J. Cell Sci. 117, 5509–5519 (2004).
Meyerholz, D. K. et al. Early epithelial invasion by Salmonella enterica serovar Typhimurium DT104 in the swine ileum. Vet. Pathol. 39, 712–720 (2002).
Ren, J. et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 5, 163–175 (2004). First demonstration that cell surface mucins modulate apoptosis in epithelial cells.
Wei, X., Xu, H. & Kufe, D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell 7, 167–178 (2005).
Kim, K. C., Lee, B. C., Pou, S. & Ciccolella, D. Effects of activation of polymorphonuclear leukocytes on airway goblet cell mucin release in a co-culture system. Inflamm Res. 52, 258–262 (2003).
Fischer, B. M., Krunkosky, T. M., Wright, D. T., Dolanokeefe, M. & Adler, K. B. Tumor necrosis factor-alpha (TNF-α) stimulates mucin secretion and gene expression in airway epithelium in vitro. Chest 107, S133–S135 (1995).
Hollande, E., Fanjul, M., Claret, S., Forguelafitte, M. E. & Bara, J. Effects of VIP on the regulation of mucin secretion in cultured human pancreatic cancer cells (Capan-1). In Vitro Cell. Dev. Biol. 31, 227–233 (1995).
Smirnova, M. G., Guo, L., Birchall, J. P. & Pearson, J. P. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell. Immunol. 221, 42–49 (2003).
Kishioka, C., Okamoto, K., Kim, J. & Rubin, B. K. Regulation of secretion from mucous and serous cells in the excised ferret trachea. Respir. Physiol. 126, 163–171 (2001).
Smirnova, M. G., Birchall, J. P. & Pearson, J. P. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine 12, 1732–1736 (2000).
Enss, M. L. et al. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res. 49, 162–169 (2000).
Klinkspoor, J. H. et al. Mucin secretion by the human colon cell line LS174T is regulated by bile salts. Glycobiology 9, 13–19 (1999).
Voynow, J. A. et al. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 20, 835–843 (1999).
Jarry, A., Vallette, G., Branka, J. E. & Laboisse, C. Direct secretory effect of interleukin-1 via type I receptors in human colonic mucous epithelial cells (HT29-C1.16E). Gut 38, 240–242 (1996).
Kim, K. C., Park, H. R., Shin, C. Y., Akiyama, T. & Ko, K. H. Nucleotide-induced mucin release from primary hamster tracheal surface epithelial cells involves the P2u purinoceptor. Eur. Resp. J. 9, 542–548 (1996).
Gottke, M. & Chadee, K. Exogenous nitric oxide stimulates mucin secretion from LS174T colonic adenocarcinoma cells. Inflamm. Res. 45, 209–212 (1996).
Tam, P. Y. & Verdugo, P. Control of mucus hydration as a Donnan equilibrium process. Nature 292, 340–342 (1981).
Verdugo, P. Mucin exocytosis. Am. Rev. Respir. Dis. 144, S33–S37 (1991).
Hill, R. R., Cowley, H. M. & Andremont, A. Influence of colonizing micro-flora on the mucin histochemistry of the neonatal mouse colon. Histochem. J. 22, 102–105 (1990).
Enss, M. L. et al. Response of germfree rat colonic mucous cells to peroral endotoxin application. Eur. J. Cell Biol. 71, 99–104 (1996).
Kandori, H., Hirayama, K., Takeda, M. & Doi, K. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp. Anim. 45, 155–160 (1996).
Fukushima, K. et al. Colonization of microflora in mice: mucosal defense against luminal bacteria. J. Gastroenterol. 34, 54–60 (1999).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006). Finding that the production of specific antimicrobial molecules is regulated by the commensal microbiota.
Hooper, L. V., Stappenbeck, T. S., Hong, C. V. & Gordon, J. I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nature Immunol. 4, 269–273 (2003).
Sharma, R. & Schumacher, U. Carbohydrate expression in the intestinal mucosa. Adv. Anat. Embryol. Cell Biol. 160, III–IX, 1–91 (2001).
Freitas, M., Axelsson, L. G., Cayuela, C., Midtvedt, T. & Trugnan, G. Microbial-host interactions specifically control the glycosylation pattern in intestinal mouse mucosa. Histochem. Cell Biol. 118, 149–161 (2002).
George, S. et al. Lectin binding profile of the small intestine of five-week-old pigs in response to the use of chlortetracycline as a growth promotant and under gnotobiotic conditions. J. Anim. Sci. 85, 1640–1650 (2007).
Whittaker, L. et al. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am. J. Respir. Cell. Mol. Biol. 27, 593–602 (2002).
Artis, D. et al. Tumor necrosis factor α is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J. Exp. Med. 190, 953–962 (1999).
Park, K. S. et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117, 978–988 (2007). First demonstration of the importance of the T H 2 cytokine mediated production of the SPDEF transcription factor on goblet cell differentiation.
Gregorieff, A. et al. The Ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 137, 1333–1345.e3 (2009).
Noah, T. K., Kazanjian, A., Whitsett, J. & Shroyer, N. F. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp. Cell Res. 316, 452–465 (2009).
Chen, G. et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914–2924 (2009).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Chen, Y. et al. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 278, 17036–17043 (2003).
Andrianifahanana, M. et al. IFN-γ-induced expression of MUC4 in pancreatic cancer cells is mediated by STAT-1 upregulation: a novel mechanism for IFN-γ response. Oncogene 26, 7251–7261 (2007).
Ahn, D. H. et al. TNF-alpha activates MUC2 transcription via NF-kappaB but inhibits via JNK activation. Cell Physiol. Biochem. 15, 29–40 (2005).
Dabbagh, K. et al. IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J.Immunol. 162, 6233–6237 (1999).
Longphre, M. et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J. Clin. Invest. 104, 1375–1382 (1999).
Kim, Y. D. et al. Regulation of IL-1β-mediated MUC2 gene in NCI-H292 human airway epithelial cells. Biochem. Biophys. Res. Commun. 274, 112–116 (2000).
Shim, J. J. et al. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L134–L140 (2001).
Smirnova, M. G., Kiselev, S. L., Birchall, J. P. & Pearson, J. P. Up-regulation of mucin secretion in HT29-MTX cells by the pro-inflammatory cytokines tumor necrosis factor-α and interleukin-6. Eur. Cytokine Netw. 12, 119–125 (2001).
Kim, Y. D. et al. Interleukin-1β induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J. Korean Med. Sci. 17, 765–771 (2002).
Song, J. S. et al. Nitric oxide induces MUC5AC mucin in respiratory epithelial cells through PKC and ERK dependent pathways. Respir. Res. 8, 28 (2007).
Wu, Y. M., Nowack, D. D., Omenn, G. S. & Haab, B. B. Mucin glycosylation is altered by pro-inflammatory signaling in pancreatic-cancer cells. J. Proteome Res. 8, 1876–1886 (2009).
Kanoh, A. et al. Interleukin-4 induces specific pp-GalNAc-T expression and alterations in mucin O-glycosylation in colonic epithelial cells. Biochim. Biophys. Acta 1780, 577–584 (2008).
Groux-Degroote, S. et al. IL-6 and IL-8 increase the expression of glycosyltransferases and sulfotransferases involved in the biosynthesis of sialylated and/or sulfated LewisX epitopes in the human bronchial mucosa. Biochem. J. 410, 213–223 (2008).
Beum, P. V., Basma, H., Bastola, D. R. & Cheng, P. W. Mucin biosynthesis: upregulation of core 2 β1,6 N-acetylglucosaminyltransferase by retinoic acid and Th2 cytokines in a human airway epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L116–L124 (2005).
Yamauchi, J. et al. Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection with the nematode Nippostrongylus brasiliensis in rat. APMIS 114, 270–278 (2006).
Takeda, K. et al. Direct effects of IL-4/IL-13 and the nematode Nippostrongylus brasiliensis on intestinal epithelial cells in vitro. Parasite Immunol. 32, 420–429 (2010).
Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J. & Madara, J. L. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 (2001).
Yanagihara, K., Seki, M. & Cheng, P. W. Lipopolysaccharide induces mucus cell metaplasia in mouse lung. Am. J. Respir. Cell. Mol. Biol. 24, 66–73 (2001).
Lemjabbar, H. & Basbaum, C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nature Med. 8, 41–46 (2002).
Dohrman, A. et al. Mucin gene (MUC2 and MUC5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta 1406, 251–259 (1998).
Caballero-Franco, C., Keller, K., De Simone, C. & Chadee, K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G315–G322 (2007).
Lievin- Le Moal, V., Servin, A. L. & Coconnier-Polter, M. H. The increase in mucin exocytosis and the upregulation of MUC genes encoding for membrane-bound mucins induced by the thiol-activated exotoxin listeriolysin O is a host cell defence response that inhibits the cell-entry of Listeria monocytogenes. Cell. Microbiol. 7, 1035–1048 (2005).
Slomiany, B. L. & Slomiany, A. Cytosolic phospholipase A2 activation in Helicobacter pylori lipopolysaccharide-induced interference with gastric mucin synthesis. IUBMB Life 58, 217–223 (2006).
Linden, S. K., Florin, T. H. & McGuckin, M. A. Mucin dynamics in intestinal bacterial infection. PLoS ONE 3, e3952 (2008). Description of progressive changes in cell surface and secreted intestinal mucins during infection with an attaching and effacing pathogen.
Boshuizen, J. A. et al. Homeostasis and function of goblet cells during rotavirus infection in mice. Virology 337, 210–221 (2005).
Hasnain, S. Z., Thornton, D. J. & Grencis, R. K. Changes in the mucosal barrier during acute and chronic Trichuris muris infection. Parasite Immunol. 33, 45–55 (2011).
Guk, S. M. et al. CD4+ T-cell-dependent goblet cell proliferation and expulsion of Gymnophalloides seoi from the intestine of C57BL/6 mice. J. Parasitol. 95, 581–590 (2009).
Hoang, V. C., Williams, M. A. & Simpson, H. V. Effects of weaning and infection with Teladorsagia circumcincta on mucin carbohydrate profiles of early weaned lambs. Vet. Parasitol. 171, 354–360 (2010).
Li, R. W. et al. Mucin biosynthesis in the bovine goblet cell induced by Cooperia oncophora infection. Vet. Parasitol. 165, 281–289 (2009).
Fontaine, O. et al. Setting research priorities to reduce global mortality from childhood diarrhoea by 2015. PLoS Med. 6, e41 (2009).
Linden, S. K., Driessen, K. M. & McGuckin, M. A. Improved in vitro model systems for gastrointestinal infection by choice of cell line, pH, microaerobic conditions and optimization of culture conditions. Helicobacter 12, 341–353 (2007).
Wickstrom, C., Herzberg, M. C., Beighton, D. & Svensater, G. Proteolytic degradation of human salivary MUC5B by dental biofilms. Microbiology 155, 2866–2872 (2009).
Tsai, H. H., Dwarakanath, A. D., Hart, C. A., Milton, J. D. & Rhodes, J. M. Increased faecal mucin sulphatase activity in ulcerative colitis: a potential target for treatment. Gut 36, 570–576 (1995).
Corfield, A. P. et al. The roles of enteric bacterial sialidase, sialate O-acetyl esterase and glycosulfatase in the degradation of human colonic mucin. Glycoconj. J. 10, 72–81 (1993).
Roberton, A. M. et al. A novel bacterial mucinase, glycosulfatase, is associated with bacterial vaginosis. J. Clin. Microbiol. 43, 5504–5508 (2005).
Berry, M., Harris, A., Lumb, R. & Powell, K. Commensal ocular bacteria degrade mucins. Br. J. Ophthalmol. 86, 1412–1416 (2002).
Martens, E. C., Roth, R., Heuser, J. E. & Gordon, J. I. Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J. Biol. Chem. 284, 18445–18457 (2009).
Falk, P., Hoskins, L. C. & Larson, G. Bacteria of the human intestinal microbiota produce glycosidases specific for lacto-series glycosphingolipids. J. Biochem. 108, 466–474 (1990); erratum 109, 798 (1991).
Vanderhoeven, J. S. & Camp, P. J. M. The use of lectins in monitoring degradation of oligosaccharide chains in mucin by oral streptococci. Caries Res. 28, 257–261 (1994).
Jansen, H. J., Hart, C. A., Rhodes, J. M., Saunders, J. R. & Smalley, J. W. A novel mucin-sulphatase activity found in Burkholderia cepacia and Pseudomonas aeruginosa. J. Med. Microbiol. 48, 551–557 (1999).
Henderson, I. R., Czeczulin, J., Eslava, C., Noriega, F. & Nataro, J. P. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67, 5587–5596 (1999).
Slomiany, B. L. et al. Campylobacter pyloridis degrades mucin and undermines gastric mucosal integrity. Biochem. Biophys. Res. Commun. 144, 307–314 (1987).
Terra, V. S., Homer, K. A., Rao, S. G., Andrew, P. W. & Yesilkaya, H. Characterization of novel β-galactosidase activity that contributes to glycoprotein degradation and virulence in Streptococcus pneumoniae. Infect. Immun. 78, 348–357 (2010).
Yesilkaya, H., Manco, S., Kadioglu, A., Terra, V. S. & Andrew, P. W. The ability to utilize mucin affects the regulation of virulence gene expression in Streptococcus pneumoniae. FEMS Microbiol. Lett. 278, 231–235 (2008).
Mantle, M. & Rombough, C. Growth in and breakdown of purified rabbit small intestinal mucin by Yersinia enterocolitica. Infect. Immun. 61, 4131–4138 (1993).
Ashida, H. et al. Characterization of two different endo-α-N-acetylgalactosaminidases from probiotic and pathogenic enterobacteria, Bifidobacterium longum and Clostridium perfringens. Glycobiology 18, 727–734 (2008).
Szabady, R. L., Yanta, J. H., Halladin, D. K., Schofield, M. J. & Welch, R. A. TagA is a secreted protease of Vibrio cholerae that specifically cleaves mucin glycoproteins. Microbiology 157, 516–525 (2011).
Grys, T. E., Walters, L. L. & Welch, R. A. Characterization of the StcE protease activity of Escherichia coli O157:H7. J. Bacteriol. 188, 4646–4653 (2006).
Cervantes-Sandoval, I., Serrano-Luna Jde, J., Garcia-Latorre, E., Tsutsumi, V. & Shibayama, M. Mucins in the host defence against Naegleria fowleri and mucinolytic activity as a possible means of evasion. Microbiology 154, 3895–3904 (2008).
Acknowledgements
M.A.M. and T.H.F. are supported by an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellowship and an NHMRC Senior Practitioner Fellowship, respectively. This work is supported by NHMRC project grant 543704.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Commensal microorganism
-
A microorganism living in a mutually advantageous relationship with a mammalian host (for example, in the lumen of the gastrointestinal tract).
- Mucus
-
A viscous, highly hydrated fluid layer that overlies the mucosal surface and is rich in secreted mucin glycoproteins and other molecules involved in host defence against infection.
- Mucin
-
A cell surface or secreted glycoprotein that is characterized by the presence of a large filamentous domain which is rich in complex O-linked oligosaccharides.
- Antimicrobial molecule
-
One of a group of molecules that are produced by Paneth cells and other epithelial cells and that have a wide variety of structures. Many are small, cationic and amphipathic peptides or lectins, and are microbicidal because they interact with and disrupt microbial cell membranes. Many of these molecules can also be produced by granulocytes.
- Secreted mucin
-
A secreted glycoprotein with a central domain containing a dense array of O-linked oligosaccharides, and amino- and carboxy-terminal cysteine-rich domains that oligomerize the mucins into a large macromolecular complex, giving mucus its viscous properties.
- Goblet cell
-
A specialized secretory cell that manufactures secreted mucin glycoproteins and other components of the mucus.
- Paneth cell
-
A specialized intestinal secretory cell that manufactures antimicrobial molecules for secretion into the mucus.
- Theca
-
An intracellular collection of mucin granules that are packaged for secretion; the theca is responsible for the classical goblet cell morphology that is seen following conventional cell fixation.
- Lewis blood group antigen
-
A carbohydrate antigen that is found on the cell surface of red blood cells and also on epithelial glycolipids and glycoproteins, including mucins. Polymorphisms in glycosyltransferases determine the presence or absence of Lewis carbohydrates and also the precise isoforms of carbohydrate that are expressed on blood cells and the mucosae.
- Lamina propria
-
The tissue that underlies the mucosal epithelium and contains immune cells, connective tissue, and blood and lymphatic vessels.
- Rheological
-
Pertaining to the study of the properties of flow of matter.
- Cell surface mucin
-
A transmembrane glycoprotein with a dense array of O-linked oligosaccharides in its extracellular domain and with complex cytoplasmic domains that are involved in intracellular signalling. The extracellular domain of these mucins can be shed from the cell surface.
- Glycocalyx
-
An extracellular zone on the apical surface of mucosal epithelial cells that is composed of carbohydrate-rich transmembrane and secreted molecules, including cell surface mucin glycoproteins.
- Peyer's patch
-
An organized collection of tertiary lymphoid tissue directly underlying the intestinal epithelium; mainly found in the small intestine.
- Transcytose
-
To pass something (such as a microorganism) through a cell.
Rights and permissions
About this article
Cite this article
McGuckin, M., Lindén, S., Sutton, P. et al. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9, 265–278 (2011). https://doi.org/10.1038/nrmicro2538
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2538
This article is cited by
-
Immunity to Cryptosporidium: insights into principles of enteric responses to infection
Nature Reviews Immunology (2024)
-
State of the art in research on the gut-liver and gut-brain axis in poultry
Journal of Animal Science and Biotechnology (2023)
-
miR-125a-5p regulates the sialyltransferase ST3GAL1 in murine model of human intestinal campylobacteriosis
Gut Pathogens (2023)
-
The protective role of conjunctival goblet cell mucin sialylation
Nature Communications (2023)
-
Basal stem cell progeny establish their apical surface in a junctional niche during turnover of an adult barrier epithelium
Nature Cell Biology (2023)