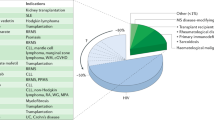
Abstract
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the brain caused by the JC virus (JCV). PML usually occurs via reactivation of JCV when an immune system becomes compromised. A diagnosis of PML is normally made on the basis of distinguishing neurological features at presentation, characteristic brain MRI changes and the presence of JCV DNA in cerebrospinal fluid. PML has a 3 month mortality rate of 20–50%, so prompt intervention is essential. Currently, reconstitution of the immune system affords the best prognosis for this condition. When PML is first suspected, and where possible, immunosuppressant or immunomodulatory therapy should be suspended or reduced. If PML is associated with a protein therapy that has a long half-life the use of plasma exchange to accelerate the removal of the drug from the circulation may aid the restoration of immune system function. Rapid improvements in immune function, however, might lead to transient worsening of the disease. In this Review, we critically appraise the controversies surrounding JCV infection, and provide practical management guidelines for PML.
Key Points
-
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the brain caused by the JC virus (JCV), and is predominantly associated with HIV infection and immunomodulatory therapies
-
A diagnosis of PML is based on neurological signs at presentation, an MRI scan that is consistent with PML, and the presence of JCV DNA in cerebrospinal fluid
-
When possible, a suspicion of PML should prompt suspension or reduction of immunosuppressant therapy, and the patient should have a thorough neurological assessment by a physician with neurological experience
-
Reconstitution of the immune system is associated with the best PML prognosis
-
Patients who have received immune system reconstitution therapy should be carefully monitored, as they can develop immune reconstitution inflammatory syndrome (IRIS)
-
When IRIS is suspected to be the cause of neurological deterioration, corticosteroid treatment should be considered
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Astrom, K. E., Mancall, E. L. & Richardson, E. P. Jr. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 81, 93–111 (1958).
Carson, K. R. et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 10, 816–824 (2009).
Cinque, P., Koralnik, I. J., Gerevini, S., Miro, J. M. & Price, R. W. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect. Dis. 9, 625–636 (2009).
Major, E. O., Amemiya, K., Tornatore, C. S., Houff, S. A. & Berger, J. R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 5, 49–73 (1992).
Walker, D. L. & Padgett, B. L. The epidemiology of human polyomaviruses. Prog. Clin. Biol. Res. 105, 99–106 (1983).
Safak, M. & Khalili, K. An overview: human polyomavirus JC virus and its associated disorders. J. Neurovirol. 9 (Suppl. 1), 3–9 (2003).
Markowitz, R. B., Thompson, H. C., Mueller, J. F., Cohen, J. A. & Dynan, W. S. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J. Infect. Dis. 167, 13–20 (1993).
Lednicky, J. A. et al. Polyomavirus JCV excretion and genotype analysis in HIV-infected patients receiving highly active antiretroviral therapy. AIDS 17, 801–807 (2003).
Egli, A. et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199, 837–846 (2009).
Gorelik, L. et al. Evaluation of the incidence of anti-JCV antibodies in a cohort of natalizumab-treated MS patients [abstract S31.003]. Neurology 74 (Suppl. 2), A390 (2010).
Brooks, B. R. & Walker, D. L. Progressive multifocal leukoencephalopathy. Neurol. Clin. 2, 299–313 (1984).
Gheuens, S., Pierone, G., Peeters, P. & Koralnik, I. J. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J. Neurol. Neurosurg. Psychiatry 81, 247–254 (2010).
d'Arminio Monforte, A. et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann. Neurol. 55, 320–328 (2004).
Power, C. et al. AIDS- and non-AIDS-related PML association with distinct p53 polymorphism. Neurology 54, 743–746 (2000).
Gonzalez, H., Bolgert, F., Camporo, P. & Leblond, V. Progressive multifocal leukoencephalitis (PML) in three patients treated with standard-dose fludarabine (FAMP). Hematol. Cell Ther. 41, 183–186 (1999).
Weber, T., Weber, F., Petry, H. & Luke, W. Immune response in progressive multifocal leukoencephalopathy: an overview. J. Neurovirol. 7, 311–317 (2001).
Berger, J. R., Pall, L., Lanska, D. & Whiteman, M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J. Neurovirol. 4, 59–68 (1998).
Calabrese, L. H., Molloy, E. S., Huang, D. & Ransohoff, R. M. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathologic patterns of disease. Arthritis Rheum. 56, 2116–2128 (2007).
Ahmed, F., Aziz, T. & Kaufman, L. D. Progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematosus. J. Rheumatol. 26, 1609–1612 (1999).
Pelosini, M. et al. Progressive multifocal leukoencephalopathy: report of three cases in HIV-negative hematological patients and review of literature. Ann. Hematol. 87, 405–412 (2008).
Yousry, T. A. et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 354, 924–933 (2006).
Carson, K. R. et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113, 4834–4840 (2009).
Bagel, J. Understanding PML and risks associated with biologic therapies. Practical Dermatology 6, 27–28 (2009).
Martin, S. I. et al. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin. Infect. Dis. 43, 16–24 (2006).
Clarke, T. Corrected—Biogen reports six new PML cases with Tysabri. Reuters.com [online], (2010).
Clifford, D. B. et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 9, 438–446 (2010).
Molloy, E. S. & Calabrese, L. H. Progressive multifocal leukoencephalopathy: a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum. 60, 3761–3765 (2009).
Bofill-Mas, S. & Girones, R. Role of the environment in the transmission of JC virus. J. Neurovirol. 9 (Suppl. 1), 54–58 (2003).
Liu, C. K., Wei, G. & Atwood, W. J. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal alpha(2–6)-linked sialic acids. J. Virol. 72, 4643–4649 (1998).
Elphick, G. F. et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 306, 1380–1383 (2004).
Khalili, K., White, M. K., Lublin, F., Ferrante, P. & Berger, J. R. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology 68, 985–990 (2007).
Tan, C. S. & Koralnik, I. J. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 9, 425–437 (2010).
Sabath, B. F. & Major, E. O. Traffic of JC virus from sites of initial infection to the brain: the path to progressive multifocal leukoencephalopathy. J. Infect. Dis. 186 (Suppl. 2), S180–S186 (2002).
Major, E. O. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu. Rev. Med. 61, 35–47 (2010).
Berger, J. R. Progressive multifocal leukoencephalopathy. Curr. Neurol. Neurosci. Rep. 7, 461–469 (2007).
Sunyaev, S. R., Lugovskoy, A., Simon, K. & Gorelik, L. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML). PLoS Genet. 5, e1000368 (2009).
Koralnik, I. J. Progressive multifocal leukoencephalopathy revisited: has the disease outgrown its name? Ann. Neurol. 60, 162–173 (2006).
Khanna, N. et al. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J. Virol. 83, 4404–4411 (2009).
Tada, H. et al. Trans-activation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc. Natl Acad. Sci. USA 87, 3479–3483 (1990).
Wuthrich, C. et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J. Neuropathol. Exp. Neurol. 68, 15–25 (2009).
Wuthrich, C. et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann. Neurol. 65, 742–748 (2009).
Newman, J. T. & Frisque, R. J. Detection of archetype and rearranged variants of JC virus in multiple tissues from a pediatric PML patient. J. Med. Virol. 52, 243–252 (1997).
Houff, S. A. et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N. Engl. J. Med. 318, 301–305 (1988).
Ciappi, S. et al. Archetypal and rearranged sequences of human polyomavirus JC transcription control region in peripheral blood leukocytes and in cerebrospinal fluid. J. Gen. Virol. 80, 1017–1023 (1999).
Fedele, C. G. et al. Identical rearranged forms of JC polyomavirus transcriptional control region in plasma and cerebrospinal fluid of acquired immunodeficiency syndrome patients with progressive multifocal leukoencephalopathy. J. Neurovirol. 9, 551–558 (2003).
Pfister, L. A., Letvin, N. L. & Koralnik, I. J. JC virus regulatory region tandem repeats in plasma and central nervous system isolates correlate with poor clinical outcome in patients with progressive multifocal leukoencephalopathy. J. Virol. 75, 5672–5676 (2001).
Koralnik, I. J., Boden, D., Mai, V. X., Lord, C. I. & Letvin, N. L. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology 52, 253–260 (1999).
Perez-Liz, G., Del Valle, L., Gentilella, A., Croul, S. & Khalili, K. Detection of JC virus DNA fragments but not proteins in normal brain tissue. Ann. Neurol. 64, 379–387 (2008).
Stuve, O. et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch. Neurol. 63, 1383–1387 (2006).
Lu, T. T. & Cyster, J. G. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297, 409–412 (2002).
Arroyo, A. G., Yang, J. T., Rayburn, H. & Hynes, R. O. α4 Integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity 11, 555–566 (1999).
Krumbholz, M., Meinl, I., Kumpfel, T., Hohlfeld, R. & Meinl, E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 71, 1350–1354 (2008).
Chen, Y. et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361, 1067–1074 (2009).
Ransohoff, R. M. “Thinking without thinking” about natalizumab and PML. J. Neurol. Sci. 259, 50–52 (2007).
Chapagain, M. L., Verma, S., Mercier, F., Yanagihara, R. & Nerurkar, V. R. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology 364, 55–63 (2007).
Dubois, V. et al. Latency and reactivation of JC virus in peripheral blood of human immunodeficiency virus type 1-infected patients. J. Clin. Microbiol. 35, 2288–2292 (1997).
Branchetti, E. et al. Treatment of PBMC with the immunosuppressive drug rituximab reactivates JC virus gene expression and replication in glial cells. J. Neurovirol. 12, 12–13 (2009).
Stasi, R. et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 110, 2924–2930 (2007).
Chen, Y. et al. Subclinical reactivation of JC virus in blood and urine of multiple sclerosis patients treated with natalizumab. J. Neurovirol. 15, 16–17 (2009).
Jilek, S. et al. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol. 9, 264–272 (2010).
Warnke, C., Adams, O. & Kieseier, B. C. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361, 2489–2490 (2009).
Zunt, J. R., Tu, R. K., Anderson, D. M., Copass, M. C. & Marra, C. M. Progressive multifocal leukoencephalopathy presenting as human immunodeficiency virus type 1 (HIV)-associated dementia. Neurology 49, 263–265 (1997).
Lima, M. A., Drislane, F. W. & Koralnik, I. J. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology 66, 262–264 (2006).
Bernal-Cano, F., Joseph, J. T. & Koralnik, I. J. Spinal cord lesions of progressive multifocal leukoencephalopathy in an acquired immunodeficiency syndrome patient. J. Neurovirol. 13, 474–476 (2007).
Bergui, M. et al. Progressive multifocal leukoencephalopathy: diffusion-weighted imaging and pathological correlations. Neuroradiology 46, 22–25 (2004).
Chang, L. et al. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology 48, 836–845 (1997).
Kappos, L. et al. Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol. 6, 431–441 (2007).
Hammarin, A. L. et al. Analysis of PCR as a tool for detection of JC virus DNA in cerebrospinal fluid for diagnosis of progressive multifocal leukoencephalopathy. J. Clin. Microbiol. 34, 2929–2932 (1996).
Vago, L. et al. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12, 139–146 (1996).
Ryschkewitsch, C. et al. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J. Virol. Methods 121, 217–221 (2004).
Cinque, P., Scarpellini, P., Vago, L., Linde, A. & Lazzarin, A. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS 11, 1–17 (1997).
Clifford, D. B. et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 9, 438–446 (2010).
Landry, M. L., Eid, T., Bannykh, S. & Major, E. False negative PCR despite high levels of JC virus DNA in spinal fluid: Implications for diagnostic testing. J. Clin. Virol. 43, 247–249 (2008).
Iacobaeus, E. et al. Analysis of cerebrospinal fluid and cerebrospinal fluid cells from patients with multiple sclerosis for detection of JC virus DNA. Mult. Scler. 15, 28–35 (2009).
Alvarez-Lafuente, R., Garcia-Montojo, M., De Las Heras, V., Bartolome, M. & Arroyo, R. JC virus in cerebrospinal fluid samples of multiple sclerosis patients at the first demyelinating event. Mult. Scler. 13, 590–595 (2007).
Sindic, C. J. et al. Detection of CSF-specific oligoclonal antibodies to recombinant JC virus VP1 in patients with progressive multifocal leukoencephalopathy. J. Neuroimmunol. 76, 100–104 (1997).
Weber, T. et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J. Infect. Dis. 176, 250–254 (1997).
Knowles, W. A., Luxton, R. W., Hand, J. F., Gardner, S. D. & Brown, D. W. The JC virus antibody response in serum and cerebrospinal fluid in progressive multifocal leucoencephalopathy. Clin. Diagn. Virol. 4, 183–194 (1995).
Guillaume, B., Sindic, C. J. & Weber, T. Progressive multifocal leukoencephalopathy: simultaneous detection of JCV DNA and anti-JCV antibodies in the cerebrospinal fluid. Eur. J. Neurol. 7, 101–106 (2000).
Koralnik, I. J. New insights into progressive multifocal leukoencephalopathy. Curr. Opin. Neurol. 17, 365–370 (2004).
Adcock, J. E., Davies, M. A., Turner, T., Pell, M. & Brew, B. Progressive multifocal leukoencephalopathy: a retrospective study of 30 cases. J. Clin. Neurosci. 4, 463–468 (1997).
Dubois, V. et al. Detection of JC virus DNA in the peripheral blood leukocytes of HIV-infected patients. AIDS 10, 353–358 (1996).
Gorelik, L. et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361, 2487–2490 (2009).
Clifford, D. B. et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 52, 623–625 (1999).
Shitrit, D., Lev, N., Bar-Gil-Shitrit, A. & Kramer, M. R. Progressive multifocal leukoencephalopathy in transplant recipients. Transpl. Int. 17, 658–665 (2005).
Crowder, C. D. et al. Successful outcome of progressive multifocal leukoencephalopathy in a renal transplant patient. Am. J. Transplant. 5, 1151–1158 (2005).
Dworkin, M. S. A review of progressive multifocal leukoencephalopathy in persons with and without AIDS. Curr. Clin. Top. Infect. Dis. 22, 181–195 (2002).
Seth, P., Diaz, F. & Major, E. O. Advances in the biology of JC virus and induction of progressive multifocal leukoencephalopathy. J. Neurovirol. 9, 236–246 (2003).
Collazos, J. Opportunistic infections of the CNS in patients with AIDS: diagnosis and management. CNS Drugs 17, 869–887 (2003).
Safrin, S., Cherrington, J. & Jaffe, H. S. Clinical uses of cidofovir. Rev. Med. Virol. 7, 145–156 (1997).
O'Riordan, T., Daly, P. A., Hutchinson, M., Shattock, A. G. & Gardner, S. D. Progressive multifocal leukoencephalopathy-remission with cytarabine. J. Infect. 20, 51–54 (1990).
De Luca, A. et al. Clinical and virological monitoring during treatment with intrathecal cytarabine in patients with AIDS-associated progressive multifocal leukoencephalopathy. Clin. Infect. Dis. 28, 624–628 (1999).
Hall, C. D. et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N. Engl. J. Med. 338, 1345–1351 (1998).
Albrecht, H. et al. Highly active antiretroviral therapy significantly improves the prognosis of patients with HIV-associated progressive multifocal leukoencephalopathy. AIDS 12, 1149–1154 (1998).
Huang, S. S., Skolasky, R. L., Dal Pan, G. J., Royal, W. 3rd & McArthur, J. C. Survival prolongation in HIV-associated progressive multifocal leukoencephalopathy treated with alpha-interferon: an observational study. J. Neurovirol. 4, 324–332 (1998).
De Luca, A. et al. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS 22, 1759–1767 (2008).
Geschwind, M. D., Skolasky, R. I., Royal, W. S. & McArthur, J. C. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J. Neurovirol. 7, 353–357 (2001).
Co, J. K., Verma, S., Gurjav, U., Sumibcay, L. & Nerurkar, V. R. Interferon- alpha and -beta restrict polyomavirus JC replication in primary human fetal glial cells: implications for progressive multifocal leukoencephalopathy therapy. J. Infect. Dis. 196, 712–718 (2007).
Focosi, D., Fazzi, R., Montanaro, D., Emdin, M. & Petrini, M. Progressive multifocal leukoencephalopathy in a haploidentical stem cell transplant recipient: a clinical, neuroradiological and virological response after treatment with risperidone. Antiviral Res. 74, 156–158 (2007).
Verma, S. et al. Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J. Infect. Dis. 196, 709–711 (2007).
Chapagain, M. L. et al. Serotonin receptor 2A blocker (risperidone) has no effect on human polyomavirus JC infection of primary human fetal glial cells. J. Neurovirol. 14, 448–454 (2008).
Marzocchetti, A. et al. Efficient in vitro expansion of JC virus-specific CD8+ T-cell responses by JCV peptide-stimulated dendritic cells from patients with progressive multifocal leukoencephalopathy. Virology 383, 173–177 (2009).
Re, D. et al. Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation and alpha-interferon immunotherapy. Bone Marrow Transplant. 23, 295–298 (1999).
Kunschner, L. & Scott, T. F. Sustained recovery of progressive multifocal leukoencephalopathy after treatment with IL-2. Neurology 65, 1510 (2005).
Przepiorka, D. et al. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. 20, 983–987 (1997).
Chang, C. F. et al. Evidence that replication of human neurotropic JC virus DNA in glial cells is regulated by the sequence-specific single-stranded DNA-binding protein Pur alpha. J. Virol. 70, 4150–4156 (1996).
Abrams, D et al. Interleukin-2 therapy in patients with HIV infection. N. Engl. J. Med. 361, 1548–1559 (2009).
Brickelmaier, M. et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob. Agents Chemother. 53, 1840–1849 (2009).
[No authors listed] Study to explore the effect of mefloquine in subjects with progressive multifocal leukoencephalopathy. ClinicalTrials.gov [online], (2009).
Du Pasquier, R. A. & Koralnik, I. J. Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial? J. Neurovirol. 9 (Suppl. 1), 25–31 (2003).
Bossolasco, S. et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin. Infect. Dis. 40, 738–744 (2005).
Marzocchetti, A. et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 73, 1551–1558 (2009).
Gasnault, J., Lanoy, E., Bentata, M., Guiguet, M. & Costagliola, D. Intracerebral penetrating ART are more efficient on survival of HIV+ patients with progressive multifocal leucoencephalopathy (ANRS CO4—FHDH). Poster presented at the 16th Conference on Retroviruses and Opportunistic Infections: 8–11 February 2009, Montreal, Quebec, Canada (2009).
Gray, F. et al. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J. Neurovirol. 11 (Suppl. 3), 16–22 (2005).
Langer-Gould, A., Atlas, S. W., Green, A. J., Bollen, A. W. & Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353, 375–381 (2005).
Tan, K., Roda, R., Ostrow, L., McArthur, J. & Nath, A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 72, 1458–1464 (2009).
Martin-Blondel, G. et al. Is maraviroc beneficial in paradoxical progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome management? AIDS 23, 2545–2546 (2009).
O'Connor, P. W. et al. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology 62, 2038–2043 (2004).
TYSABRI® (natalizumab) [package insert] (Biogen Idec, 2008).
Khatri, B. O. et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 72, 402–409 (2009).
Breedveld, F. et al. Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J. Clin. Pharmacol. 47, 1119–1128 (2007).
Venkataramana, A. et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 67, 383–388 (2006).
Hernandez, B., Dronda, F. & Moreno, S. Treatment options for AIDS patients with progressive multifocal leukoencephalopathy. Expert Opin. Pharmacother. 10, 403–416 (2009).
Blick, G. et al. Successful resolution of progressive multifocal leukoencephalopathy after combination therapy with cidofovir and cytosine arabinoside. Clin. Infect. Dis. 26, 191–192 (1998).
Moreno, S. et al. Cytarabine therapy for progressive multifocal leukoencephalopathy in patients with AIDS. Clin. Infect. Dis. 23, 1066–1088 (1996).
Antinori, A. et al. Failure of cytarabine and increased JC virus-DNA burden in the cerebrospinal fluid of patients with AIDS-related progressive multifocal leukoencephalopathy. AIDS 8, 1022–1024 (1994).
Taoufik, Y. et al. Prognostic value of JC virus load in cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J. Infect. Dis. 178, 1816–1820 (1998).
Brambilla, A. M. et al. Remission of AIDS-associated progressive multifocal leukoencephalopathy after cidofovir therapy. J. Neurol. 246, 723–725 (1999).
Portilla, J. et al. Progressive multifocal leukoencephalopathy treated with cidofovir in HIV-infected patients receiving highly active anti-retroviral therapy. J. Infect. 41, 182–184 (2000).
Razonable, R. R. et al. Cidofovir treatment of progressive multifocal leukoencephalopathy in a patient receiving highly active anti-retroviral treatment. Mayo Clin. Proc. 76, 1171–1175 (2001).
Zimmermann, T. et al. Successful treatment of AIDS-related PML with CART and cidofovir. Eur. J. Med. Res. 6, 190–192 (2001).
De Luca, A. et al. Response to cidofovir after failure to antiretroviral therapy alone in AIDS-associated progressive multifocal leukoencephalopathy. Neurology 52, 891–892 (1999).
Meylan, P. R. et al. Monitoring the response of AIDS-related progressive multifocal leukoencephalopathy to CART and cidofovir by PCR for JC virus DNA in CSF. Eur. Neurol. 41, 172–174 (1999).
De Luca, A. et al. Potent anti-retroviral therapy with or without cidofovir for AIDS-associated progressive multifocal leukoencephalopathy: extended follow-up of an observational study. J. Neurovirol. 7, 364–368 (2001).
Nath, A. et al. Progression of progressive multifocal leukoencephalopathy despite treatment with beta-interferon. Neurology 66, 149–150 (2006).
Royal, W. 3rd. et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J. Neurovirol. 9, 411–419 (2003).
Buckanovich, R. J. et al. Nonmyeloablative allogeneic stem cell transplantation for refractory Hodgkin's lymphoma complicated by interleukin-2 responsive progressive multifocal leukoencephalopathy. Ann. Hematol. 81, 410–413 (2002).
Acknowledgements
N. W. S. Davies receives funding from the Peel Medical Trust. Laurie Barclay, freelance writer and reviewer, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
B. J. Brew, N. W. S. Davies and A. Nath researched the data for the article, provided substantial contributions to discussions of the content, and contributed to writing the article and to review and editing of the manuscript before submission. D. B. Clifford researched the data for the article and provided substantial contributions to discussions of the content, and contributed to review and editing of the manuscript before submission. P. Cinque researched the data for the article and provided substantial contributions to discussions of the content.
Corresponding author
Ethics declarations
Competing interests
B. J. Brew has received honoraria from Biogen Idec, GlaxoSmithKline for consulting. He has also received research funding from GlaxoSmithKline and NeurogesX. P. Cinque has received honoraria from Biogen Idec, GlaxoSmithKline for lectures. She has also received research funding from Biogen Idec. D. B. Clifford has received honoraria from Biogen Idec, Elan, Genentech, Genzyme, GlaxoSmithKline, Millennium Pharmaceuticals, Novartis, Roche, Schering-Plough for consulting. He has also received research funding from Bavarian Nordic, Centocor Ortho Biotech, NeurogesX, Novartis, Pfizer, Savient Pharmaceuticals, Schering-Plough and Tibotec. A. Nath has received honoraria from Biogen Idec for consulting. N. W. S. Davies declares no competing interests.
Rights and permissions
About this article
Cite this article
Brew, B., Davies, N., Cinque, P. et al. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol 6, 667–679 (2010). https://doi.org/10.1038/nrneurol.2010.164
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2010.164
This article is cited by
-
Atypical presentations and course of JC virus infection
Journal of NeuroVirology (2022)
-
Successful treatment of non-HIV progressive multifocal leukoencephalopathy: case report and literature review
Journal of Neurology (2020)
-
Approach to an Intracranial Mass in Patients With HIV
Current Neurology and Neuroscience Reports (2020)
-
Improving detection of JC virus by ultrafiltration of cerebrospinal fluid before polymerase chain reaction for the diagnosis of progressive multifocal leukoencephalopathy
BMC Neurology (2019)
-
Progressive multifocal leukoencephalopathy in a renal transplant patient
Journal of NeuroVirology (2019)