Abstract
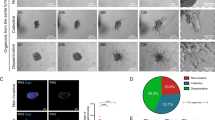
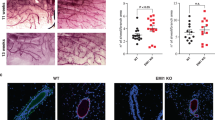
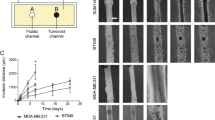
The HER2/neu oncogene encodes a receptor-like tyrosine kinase whose overexpression in breast cancer predicts poor prognosis and resistance to conventional therapies. However, the mechanisms underlying aggressiveness of HER2 (human epidermal growth factor receptor 2)-overexpressing tumors remain incompletely understood. Because it assists epidermal growth factor (EGF) and neuregulin receptors, we overexpressed HER2 in MCF10A mammary cells and applied growth factors. HER2-overexpressing cells grown in extracellular matrix formed filled spheroids, which protruded outgrowths upon growth factor stimulation. Our transcriptome analyses imply a two-hit model for invasive growth: HER2-induced proliferation and evasion from anoikis generate filled structures, which are morphologically and transcriptionally analogous to preinvasive patients’ lesions. In the second hit, EGF escalates signaling and transcriptional responses leading to invasive growth. Consistent with clinical relevance, a gene expression signature based on the HER2/EGF-activated transcriptional program can predict poorer prognosis of a subgroup of HER2-overexpressing patients. In conclusion, the integration of a three-dimensional cellular model and clinical data attributes progression of HER2-overexpressing lesions to EGF-like growth factors acting in the context of the tumor's microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
28 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41388-024-02990-w
References
Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N et al. (2003). Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res 63: 1657–1666.
Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP et al. (2006). Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 8:1235–1245.
Balleine RL, Webster LR, Davis S, Salisbury EL, Palazzo JP, Schwartz GF et al. (2008). Molecular grading of ductal carcinoma in situ of the breast. Clin Cancer Res 14: 8244–8252.
Chen DT, Nasir A, Culhane A, Venkataramu C, Fulp W, Rubio R et al. (2010). Proliferative genes dominate malignancy-risk gene signature in histologically-normal breast tissue. Breast Cancer Res Treat 119: 335–346.
Collins NL, Reginato MJ, Paulus JK, Sgroi DC, Labaer J, Brugge JS . (2005). G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression. Mol Cell Biol 25: 5282–5291.
Cox DR . (1972). Regression models and life tables. J R Statist Soc B 4: 102–118.
Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS . (2002). The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111: 29–40.
Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G et al. (2008a). Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 14: 5158–5165.
Desmedt C, Ruiz-Garcia E, Andre F . (2008b). Gene expression predictors in breast cancer: current status, limitations and perspectives. Eur J Cancer 44: 2714–2720.
Ensslin MA, Shur BD . (2007). The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis. Proc Natl Acad Sci USA 104: 2715–2720.
Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT et al. (2006). Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440: 1222–1226.
Erler JT, Weaver VM . (2009). Three-dimensional context regulation of metastasis. Clin Exp Metastasis 26: 35–49.
Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V et al. (2009). A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 15: 68–74.
Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G et al. (2006). Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126: 489–502.
Haibe-Kains B, Desmedt C, Piette F, Buyse M, Cardoso F, Van't Veer L et al. (2008). Comparison of prognostic gene expression signatures for breast cancer. BMC Genomics 9: 394.
Heagerty PJ, Lumley T, Pepe MS . (2000). Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 65: 337–344.
Huang da W, Sherman BT, Lempicki RA . (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57.
Jechlinger M, Podsypanina K, Varmus H . (2009). Regulation of transgenes in three-dimensional cultures of primary mouse mammary cells demonstrates oncogene dependence and identifies cells that survive deinduction. Genes Dev 23: 1677–1688.
Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM et al. (2002). A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res 62: 4478–4483.
Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M et al. (1999). The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci USA 96: 4995–5000.
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906.
Lonardo F, Di Marco E, King CR, Pierce JH, Segatto O, Aaronson SA et al. (1990). The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. New Biol 2: 992–1003.
Lu J, Guo H, Treekitkarnmongkol W, Li P, Zhang J, Shi B et al. (2009). 14-3-3zeta Cooperates with ErbB2 to promote ductal carcinoma in situ progression to invasive breast cancer by inducing epithelial-mesenchymal transition. Cancer Cell 16: 195–207.
Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D et al. (2007). Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci USA 104: 6740–6745.
Muller WJ, Arteaga CL, Muthuswamy SK, Siegel PM, Webster MA, Cardiff RD et al. (1996). Synergistic interaction of the Neu proto-oncogene product and transforming growth factor alpha in the mammary epithelium of transgenic mice. Mol Cell Biol 16: 5726–5736.
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS . (2001). ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 3: 785–792.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al. (2000). Molecular portraits of human breast tumours. Nature 406: 747–752.
Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ . (1992). Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 89: 9064–9068.
Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L et al. (1996). Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J 15: 2452–2467.
Pontier SM, Huck L, White DE, Rayment J, Sanguin-Gendreau V, Hennessy B et al. (2010). Integrin-linked kinase has a critical role in ErbB2 mammary tumor progression: implications for human breast cancer. Oncogene 29: 3374–3385.
Riese II DJ, van Raaij TM, Plowman GD, Andrews GC, Stern DF . (1995). The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol 15: 5770–5776.
Rilke F, Colnaghi MI, Cascinelli N, Andreola S, Baldini MT, Bufalino R et al. (1991). Prognostic significance of HER-2/neu expression in breast cancer and its relationship to other prognostic factors. Int J Cancer 49: 44–49.
Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK et al. (2004). Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci USA 101: 1257–1262.
Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC et al. (2006). The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol 24: 1151–1161.
Simpson CD, Anyiwe K, Schimmer AD . (2008). Anoikis resistance and tumor metastasis. Cancer Lett 272: 177–185.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL . (1987). Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182.
Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL et al. (2006). Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin 56: 168–183.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550.
Tagliabue E, Agresti R, Carcangiu ML, Ghirelli C, Morelli D, Campiglio M et al. (2003). Role of HER2 in wound-induced breast carcinoma proliferation. Lancet 362: 527–533.
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C . (2007). An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol 8: R157.
Therneau TM, Grambsch PM . (2000). Modeling Survival Data: Extending the Cox Model. Springer Verlag: New York.
Ueda Y, Wang S, Dumont N, Yi JY, Koh Y, Arteaga CL . (2004). Overexpression of HER2 (erbB2) in human breast epithelial cells unmasks transforming growth factor beta-induced cell motility. J Biol Chem 279: 24505–24513.
Valabrega G, Montemurro F, Sarotto I, Petrelli A, Rubini P, Tacchetti C et al. (2005). TGFalpha expression impairs Trastuzumab-induced HER2 downregulation. Oncogene 24: 3002–3010.
van de Vijver MJ, Peterse JL, Mooi WJ, Wisman P, Lomans J, Dalesio O et al. (1988). Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med 319: 1239–1245.
Virolle T, Krones-Herzig A, Baron V, De Gregorio G, Adamson ED, Mercola D . (2003). Egr1 promotes growth and survival of prostate cancer cells. Identification of novel Egr1 target genes. J Biol Chem 278: 11802–11810.
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F et al. (2005). Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365: 671–679.
Zhan L, Xiang B, Muthuswamy SK . (2006). Controlled activation of ErbB1/ErbB2 heterodimers promote invasion of three-dimensional organized epithelia in an ErbB1-dependent manner: implications for progression of ErbB2-overexpressing tumors. Cancer Res 66: 5201–5208.
Acknowledgements
We thank Drs Carlos Arteaga and Ittai Ben-Porath for reagents and helpful insights. Our laboratories are supported by research grants from the National Cancer Institute (Grant CA72981), the M.D. Moross Institute for Cancer Research, Kekst Family Institute for Medical Genetics, Women's Health Research Center funded by Bennett-Pritzker Endowment Fund, Marvelle Koffler Program for Breast Cancer Research, Harry and Jeanette Weinberg Women's Health Research Endowment, Oprah Winfrey Biomedical Research Fund, Arresto Biosciences, the European Commission, and the German Research Foundation. YY is the incumbent of the Harold and Zelda Goldenberg Professorial Chair. ED is the incumbent of the Henry J Leir Professorial Chair.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pradeep, CR., Zeisel, A., Köstler, W. et al. Modeling invasive breast cancer: growth factors propel progression of HER2-positive premalignant lesions. Oncogene 31, 3569–3583 (2012). https://doi.org/10.1038/onc.2011.547
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.547
Keywords
This article is cited by
-
Development and validation of a clinicoradiomic nomogram to assess the HER2 status of patients with invasive ductal carcinoma
BMC Cancer (2022)
-
Transcriptome and genome evolution during HER2-amplified breast neoplasia
Breast Cancer Research (2021)
-
Klf5 acetylation regulates luminal differentiation of basal progenitors in prostate development and regeneration
Nature Communications (2020)
-
Reduced Basal Nitric Oxide Production Induces Precancerous Mammary Lesions via ERBB2 and TGFβ
Scientific Reports (2019)
-
Molecular and Transcriptional Signatures for ErbB2-Induced Invasion
Current Pharmacology Reports (2019)