Abstract
The clinical significance of ERBB2 amplification/overexpression in gastric cancer remains unclear. In this study, we evaluated the ERBB2 status in 463 gastric carcinomas using immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH), and compared the findings with histopathological characteristics and with disease-specific survival. ERBB2 overexpression (2+ and 3+) and amplification (ratio ERBB2/CEP17⩾2) were found in 43 (9.3%) and 38 (8.2%) gastric carcinomas, respectively. Perfect IHC/FISH correlation was found for the 19 cases scored as 0 (all negative by FISH), and also for the 25 cases scored as 3+ (all positive by FISH). One out of six carcinomas scored as 1+ and 12 out of 18 carcinomas scored as 2+ were positive by FISH. ERBB2 amplification was associated with gastric carcinomas of intestinal type (P=0.007) and with an expansive growth pattern (P=0.021). ERBB2 amplification was detected in both histological components of two mixed carcinomas, indicating a common clonal origin. A statistically significant association was found between ERBB2 amplification and worse survival in patients with expansive gastric carcinomas (P=0.011). We conclude that ERBB2 status may have clinical significance in subsets of gastric cancer patients, and that further studies are warranted to evaluate whether patients whose gastric carcinomas present ERBB2 amplification/overexpression may benefit from therapy targeting this surface receptor.
Similar content being viewed by others
Main
Despite the trend for decreasing incidence, gastric adenocarcinoma is still the second cause of cancer death worldwide (Parkin et al, 2005). The overall 5-year survival rate of patients with resectable gastric cancer ranges from 10 to 30% (Harrison et al, 1998; Msika et al, 2000; Green et al, 2002). Apart from surgical resection, evaluation of available therapies, both neo-adjuvant and adjuvant, provides conflicting results regarding the clinical outcome. Several meta-analyses have been published in an attempt to address the discrepancies reported in the literature, but recommendation for adjuvant chemotherapy in Western centres is still not consensual (Hermans et al, 1993; Earle and Maroun, 1999; Mari et al, 2000; Gianni et al, 2001; Janunger et al, 2001, 2002; Hu et al, 2002). The most important prognostic factor established for gastric cancer is the TNM stage, which is determined by the depth of invasion, involvement of lymph nodes, and distant metastasis. However, clinical outcome varies among patients in the same stage (Park et al, 2006). Therefore prognostic factors other than the TNM stage, as well as new therapies, would be of great value for gastric cancer patients.
The ERBB2 gene maps to 17q12–q21 and encodes a 185-kDa transmembrane tyrosine kinase receptor (p185), which is a member of the epidermal growth factor receptor family (Xu et al, 1984; Akiyama et al, 1986; Popescu et al, 1989). In breast carcinomas, ERBB2 functions as an oncogene, as amplification of the gene induces protein overexpression in the cell membrane (Slamon et al, 1989). Besides being a poor prognosis marker (Dowsett et al, 2003), ERBB2 amplification is a predictive marker for targeted therapy with the monoclonal antibody trastuzumab in breast cancer patients with metastatic disease (Slamon et al, 2001). More recently, trastuzumab was shown to be effective as adjuvant treatment in breast-carcinoma patients with ERBB2 amplification/overexpression (Piccart-Gebhart et al, 2005; Romond et al, 2005).
Apart from breast cancer, ERBB2 amplification has been found in other malignant tumours, such as ovarian (McKenzie et al, 1993), lung (Hirashima et al, 2001), colon (Cohen et al, 1989), and gastric carcinomas (David et al, 1992). Earlier studies in gastric carcinomas reported ERBB2 overexpression using immunohistochemistry (IHC) in 5.2–22.6% of the cases, whereas the proportion with ERBB2 amplification evaluated using fluorescence in situ hybridisation (FISH) ranged from 3.8 to 12.2% (Takehana et al, 2002; Varis et al, 2004; Park et al, 2006; Kim et al, 2007). However, the clinical significance of ERBB2 amplification/overexpression in gastric cancer patients is still not clear, because most studies had neither follow-up data nor statistical power for that purpose (David et al, 1992; Nakajima et al, 1999; Takehana et al, 2002; Kimura et al, 2004; Kim et al, 2007).
Recently, Tanner et al (2005) showed that the gastric-cancer cell line N87 presenting ERBB2 amplification is as sensitive to trastuzumab as the ERBB2-amplified breast-carcinoma cell line SKBR-3, which is a widely used reference in trastuzumab sensitivity studies (Pegram et al, 1997, 1999). Furthermore, Rebischung et al (2005) reported a case of a gastric cancer patient with ERBB2 overexpression (3+) who responded to a combination of chemotherapy and trastuzumab. A large-scale clinical study is currently being carried out to compare the response to chemotherapy combined with trastuzumab vs chemotherapy alone in gastric carcinoma patients with ERBB2 amplification.
To clarify the potential clinical relevance of ERBB2 status in gastric cancer, we have characterised its overexpression and amplification in 463 patients with clinicopathological data and a follow-up time of 6–10 years.
Materials and methods
Type of study and selection of participants
A two-step study design was used to select the gastric cancer patients. First, a cross-sectional study was used to select 463 consecutive primary gastric adenocarcinoma patients who underwent gastrectomy at the Portuguese Oncology Institute—Porto (between 1996 and 2000) to assess the frequency of ERBB2 overexpression and amplification. Simultaneously, the first step selected patients with ERBB2 amplification for the second step, resulting in a retrospective prognostic cohort study (follow-up time from 1 to 133 months, mean 52.8 months), which included all patients with ERBB2 amplification (n=38) and randomly selected patients with no amplification (n=218).
The tissue specimens for IHC and FISH analyses were archival tumour samples of surgically resected gastric carcinomas from the 463 patients. Patient age at diagnosis ranged from 26 to 91 years (median, 67 years).
Variables
Clinical data were collected by a group of clinicians blinded to ERBB2 status, using a datasheet specifically developed for this study, including the following parameters: age, gender, date of and status on last follow-up, surgery type (curative or palliative according to the surgeon) and date, TNM stage, and treatment other than surgery (if any). Time to clinical outcome was considered from the date of surgery until the last clinical appointment attended, and each patient was classified under one of the following categories: alive with no evidence of disease, alive with disease, dead with no evidence of disease, and dead from disease. For histological data collection, pathologists reviewed a representative H&E-stained slide.
Immunohistochemistry
Immunohistochemistry targeting the ERBB2 protein was carried out in 4-μm-thick tissue sections. The monoclonal antibody NCL-CB11 (mouse monoclonal antibody, Novocastra Laboratories Ltd, Newcastle upon Tyne, UK), recognising the intracellular portion of the protein, was used. Tissue sections were deparaffinised followed by antigen retrieval in citrate buffer (0.01 M, pH 6.0) at high temperature (water bath at 98 °C). After blocking for non-specific binding, the primary antibody was added in a pre-standardised dilution (1 out of 60), and incubated for 30 min at room temperature. A standard avidin–biotin–peroxidase complex technique was used for visualisation, with diaminobenzidine as chromogen (UltraVision Detection System Anti-Polyvalent, HRP/DAB Ready-To-Use, LabVision Corporation, Fremont, CA, USA). The tissue sections were then lightly counterstained with haematoxylin and cover-slipped.
The following scoring system was used: score 0, no membrane staining or <10% of cells stained; 1+, incomplete membrane staining in >10% of the cells; 2+, weak to moderate complete membrane staining >10% of the cells with; and 3+, strong and complete membrane staining in >10% of the cells. An appropriate positive control (ERBB2-overexpressing breast carcinoma) was included in each run and each section was analysed by a pathologist.
Fluorescence in situ hybridisation
From each gastric adenocarcinoma sample, 4-μm-thick sections of a representative tissue block were cut onto SuperFrost Plus adhesion slides. The slides were then deparaffinised in two series of xylol followed by two series of ethanol (8 min each), rinsed in 2 × SSC, and placed in a solution of NaS/CN 1 M at 80 °C for 10 min. The tissue was then digested with 6 mg ml−1 pepsin for 22 min at 37 °C, after which the slides were rinsed in 2 × SSC and dehydrated in an ethanol series. To assess ERBB2 amplification, a commercial probe (QBiogene, Montreal, Canada now MP Biomedicals, Irvine, CA, USA) targeting ERBB2, direct-labelled with rhodamine, and a control probe for chromosome 17 centromere (CEP17), direct-labelled with fluorescein, were used. The slides and probes were placed in a HYBrite denaturation/hybridisation system and co-denatured at 80 °C for 7 min. Hybridisation carried out for 18 h at 37 °C, followed by post-hybridisation washes in 2 × SSC/0.5% Igepal at 73 °C for 5 min and 2 × SSC/0.1% Igepal at room temperature, after which slides were counterstained with DAPI. Fluorescent images were sequentially captured with a Cohu 4900 CCD camera, using an automated filter wheel coupled to a Zeiss Axioplan fluorescence microscope and a CytoVision system.
Gene amplification was scored when a minimum of 60 cancer cell nuclei exhibited a ratio ERBB2 / CEP17 ≥2, or when an ERBB2 signal cluster was observed.
Statistical analysis
Categorical data were analysed using χ2-square test. For parametric data, Student's t-test was used when comparing two means. Survival curves were calculated according to the Kaplan–Meier method. Cases lost to follow-up and deaths caused by reasons other than gastric cancer were censored during survival analysis. The significance of differences between survival curves was determined using the log-rank or Breslow's tests. All statistical analyses were conducted using SPSS v.15 (SPSS, Chicago, IL, USA).
Results
Overexpression of ERBB2 protein
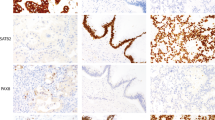
The ERBB2 protein status was determined by IHC for the 463 gastric carcinoma tissues (Figure 1). In all, 414 were classified as score 0 (89.4%), 6 were classified as score 1+ (1.3%), 18 were classified as score 2+ (3.9%), and 25 were classified as score 3+ (5.4%).
ERBB2 protein expression evaluated by IHC in gastric carcinomas. (A) Negative ERBB2 expression (0) (original magnification × 400); (B) ERBB2 positive expression –(3+) (original magnification × 400); (C) mixed type carcinoma with ERBB2 overexpression in both histological components (original magnification × 100).
ERBB2 gene amplification
Fluorescence in situ hybridisation analysis was performed in all cases (n=43) in which IHC showed complete membrane immunostaining (2+ and 3+). In addition, 25 cases (including all six 1+ cases) that were regarded as negative for ERBB2 overexpression by IHC were also analysed by FISH. Gene amplification was detected in 38 gastric carcinomas (Figure 2). Only one of the 25 tumours showing negative immunostaining (scored as +1) exhibited ERBB2 amplification (Figure 2). On the other hand, all 25 cases showing strong complete membrane immunostaining (3+) exhibited ERBB2 amplification. Among the 18 tumours with 2+ immunostaining, 12 (66.6%) showed amplification (Figure 2). Of the remaining 2+ cases, four showed chromosome 17 polysomy and two showed no genetic alteration.
Fluorescence in situ hybridisation targeting ERBB2 in gastric carcinoma specimens (original magnification, × 1000). Red labelled probes target the ERBB2, green labelled probes target chromosome 17 centromere, and nuclei (blue) are stained with DAPI. (A) intestinal type carcinoma (score 0 by IHC) with no ERBB2 amplification; (B) intestinal type carcinoma (score 2+ by IHC) showing ERBB2 amplification; (C) intestinal type carcinoma (score 1+ by IHC) showing ERBB2 amplification. A full colour version of this figure is available at the British Journal of Cancer online.
Correlation between ERBB2 amplification and clinicopathological findings
Table 1 shows the clinicopathological differences observed between gastric carcinomas with or without ERBB2 amplification. Most gastric carcinomas showing ERBB2 amplification were of the intestinal/glandular type (81.6% of all positive cases, P=0.007), but this genetic alteration was also observed in diffuse/isolated cells, and solid and mixed carcinomas (7.9, 5.3, and 5.3% of positive cases, respectively). Two mixed carcinomas showed ERBB2 amplification and overexpression in the two histological components (Figure 1). ERBB2 amplification was also associated with an expansive growth pattern (P=0.021). Venous invasion, assessed through orcein staining, was not associated with ERBB2 amplification. No differences were observed between ERBB2-amplified and ERBB2 non-amplified cases in terms of age, gender, type of surgery, and clinical stage.
Survival analysis
Survival analysis was performed on 256 patients, including all 38 who showed ERBB2 amplification. Patients with ERBB2 amplification had in general, worse 10-year survival rates than those without this genetic alteration (35.3 vs 43.2%, respectively; Figure 3), although the difference was not statistically significant (P=0.222).
Survival curves of gastric cancer patients, (A) Kaplan–Meier plot for disease-specific survival of 256 gastric cancer patients according to ERBB2 amplification status. (B) Kaplan–Meier plot for disease-specific survival of 75 expansive-type gastric cancer patients according to ERBB2 amplification status.
Differences in survival were more evident when we compared similar subgroups of patients. Patients with expansive gastric carcinoma and ERBB2 amplification had a statistically significant worse survival than those without this genetic alteration (Figure 3; P=0.011). No such difference was seen in patients with infiltrative gastric carcinoma (P=0.863). Among patients with no lymph node metastases, those with ERBB2-amplified carcinomas had a trend for worse survival when compared with those without this genetic alteration (P=0.085).
Discussion
The assessment of ERBB2 status is essential for efficient selection of patients who might benefit from targeted therapy with trastuzumab or other drugs targeting this surface receptor. This therapeutic option proved useful in extending the survival of breast cancer patients, particularly when selected by FISH (Mass et al, 2005). Since then, interest in applying this approach to other malignancies with ERBB2 amplification has increased. The first goal of this study was to clarify the frequency of ERBB2 overexpression and amplification in a large series of gastric carcinoma patients (n=463). The patients in whom ERBB2 amplification was found (n=38), as well as 218 ERBB2-negative patients, were selected for survival analysis. Using clinicopathological and follow-up data from these 256 patients, we investigated whether ERBB2 amplification is a prognosis factor in different subgroups of gastric cancer patients. Given the nature of the biological material used in this study (formalin-fixed, paraffin-embedded archival tissue), the techniques chosen to assess ERBB2 protein overexpression (IHC) and ERBB2 amplification (FISH) are the most appropriate to ensure reliable and reproducible results. Moreover, both techniques allow for the specific detection of ERBB2 alteration in individual cells, while maintaining critical architectural tissue information (Pauletti et al, 2000).
Both overexpression (9.3%) and amplification (8.2%) frequencies observed in the current study are within the range reported in earlier reports. There was a perfect correlation between IHC and FISH findings in cases with strong (3+) complete membrane staining (100%), as well as in those scored as 0. Kim et al (2007) reported 22.6% of carcinomas overexpressing ERBB2 protein (2+ or 3+), but only 7.7% showed gene amplification. As in our study, the correlation was higher in cases with strong (3+) membrane staining and in those scored as 0. Takehana et al (2002) also found ERBB2 amplification in all gastric carcinoma cases scored as 3+. On the other hand, Park et al (2006) found ERBB2 amplification in only 45% of the carcinomas scored as 3+, which might be due to the low specificity of the antibody used. In this study, one of the six gastric carcinomas classified as 1+ showed ERBB2 amplification. Similarly, Kim et al (2007) found ERBB2 amplification in 4% of the 1+ gastric carcinomas analysed by FISH. Most of the studies that reported the absence of gene amplification in carcinomas classified as 1+ did not analyse all 1+ carcinomas (Takehana et al, 2002; Park et al, 2006). As for the 18 gastric carcinomas classified as 2+ in this study, 12 (66.6%) showed ERBB2 amplification and four of the remaining six presented chromosome 17 polisomy. The correlation between 2+ carcinomas and ERBB2 amplification is higher than usually reported in the literature, possibly because of better specificity of the antibody. In the light of our observations, as well as data from the literature, we recommend that all cases classified by IHC as 1+ and 2+ should be analysed by FISH to avoid false negatives and false positives, respectively.
Some genetic alterations are exclusive of given subtypes of gastric carcinomas, as exemplified by CDH1 mutations in diffuse gastric carcinomas (Becker et al, 1994; Grady et al, 2000). Several authors have earlier stated that ERBB2 amplification is an exclusive event of intestinal-type gastric carcinomas (Takehana et al, 2002; Varis et al, 2004). However, of the 38 carcinomas with amplification detected by FISH, three were diffuse and four were not classifiable by Lauren. Other authors reported that diffuse gastric carcinomas account for 5–6.2% of all ERBB2-amplified carcinomas (Tanner et al, 2005; Park et al, 2006; Kim et al, 2007), which is corroborated by our findings. Interestingly, in both Western and Eastern patients there is a correlation between gastric carcinomas with ERBB2 amplification and intestinal type: 81.6% in this study vs 86 and 84.2% reported in Eastern patients (Park et al, 2006; Kim et al, 2007).
In two mixed gastric carcinomas containing both isolated cells and glandular components (Carneiro, 1997), ERBB2 overexpression and amplification were found in both histological patterns. These data indicate that the two histological counterparts of a carcinoma have the same clonal origin and that ERBB2 amplification is an early genetic alteration acquired before other genetic and/or epigenetic alterations associated with the phenotypic divergence in these cases. This hypothesis is supported by Carvalho et al (2006), who studied by array-comparative genome hybridisation 12 mixed carcinomas. Besides finding no significant differences between different histological components of the same tumour, one of the mixed gastric carcinomas studied presented ERBB2 amplification in both histological counterparts. Furthermore, in our consecutive series of gastric cancer patients, we found ERBB2 amplification in two early gastric carcinomas (stage IA), as reported earlier in similar studies (David et al, 1992; Ooi et al, 1998; Park et al, 2006; Kim et al, 2007), further supporting the idea that ERBB2 amplification may occur at an early stage in gastric carcinogenesis.
Our findings indicate that the influence of ERBB2 amplification on patient survival may differ among histological types and pathological staging. The overall survival analysis showed little difference in prognosis according to ERBB2 status (P=0.222), which could be explained by the patient heterogeneity regarding histological and clinical characteristics. According to Ming (1977), expansive carcinomas grow by enlargement of cohesive tumour nodules or masses, with a well-defined tumour boundary. On the other hand, infiltrative carcinomas grow as dispersed, isolated cells, and clusters or small glands that show a strong invasive potential with extensive infiltration into the stroma. This infiltrative characteristic might explain why some authors found that this type of growth pattern was associated with worse prognosis (Davessar et al, 1990; Cimerman et al, 1994; Luebke et al, 2005). Our survival analysis showed that ERBB2-amplifying expansive carcinomas have worse prognosis than those with the same growth pattern lacking that genetic alteration (P=0.011), which we did not observe in infiltrating gastric carcinomas (P=0.863). Recent data published by Wolf-Yadlin et al (2006) might explain the different effect of ERBB2 amplification in carcinomas with different growth patterns. These authors suggested that ERBB2 overexpression promotes increased cell migration but has minimal effect on cell proliferation in cells stimulated by epidermal growth factor or heregulin (Wolf-Yadlin et al, 2006). Thus, ERBB2 amplification could increase cell migration in expansive carcinomas, whereas infiltrative carcinomas, which already have a strong invasive potential, do not acquire additional advantage from ERBB2 amplification. A similar reasoning may explain our observation of a trend to worse 10-year survival among ERBB2-amplified node-negative gastric carcinomas, something that was also reported by others in breast carcinomas (Pauletti et al, 2000).
By studying the largest series of gastric cancer patients so far for ERBB2 amplification and overexpression, we conclude that ERBB2 status may have clinical significance in subsets of patients and that further studies are warranted to evaluate whether gastric cancer patients whose tumours present ERBB2 amplification/overexpression may benefit from the therapy targeting this surface receptor.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T (1986) The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 232: 1644–1646
Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H (1994) E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 54: 3845–3852
Carneiro F (1997) Classification of gastric carcinomas. Curr Diag Pathol 4: 51–59
Carvalho B, Buffart TE, Reis RM, Mons T, Moutinho C, Silva P, van Grieken NC, Grabsch H, Van de Velde CJ, Ylstra B, Meijer GA, Carneiro F (2006) Mixed gastric carcinomas show similar chromosomal aberrations in both their diffuse and glandular components. Cell Oncol 28: 283–294
Cimerman M, Repse S, Jelenc F, Omejc M, Bitenc M, Lamovec J (1994) Comparison of Lauren's, Ming's and WHO histological classifications of gastric cancer as a prognostic factor for operated patients. Int Surg 79: 27–32
Cohen JA, Weiner DB, More KF, Kokai Y, Williams WV, Maguire Jr HC, LiVolsi VA, Greene MI (1989) Expression pattern of the neu (NGL) gene-encoded growth factor receptor protein (p185neu) in normal and transformed epithelial tissues of the digestive tract. Oncogene 4: 81–88
Davessar K, Pezzullo JC, Kessimian N, Hale JH, Jauregui HO (1990) Gastric adenocarcinoma: prognostic significance of several pathologic parameters and histologic classifications. Hum Pathol 21: 325–332
David L, Seruca R, Nesland JM, Soares P, Sansonetty F, Holm R, Borresen AL, Sobrinho-Simoes M (1992) c-erbB-2 expression in primary gastric carcinomas and their metastases. Mod Pathol 5: 384–390
Dowsett M, Bartlett J, Ellis IO, Salter J, Hills M, Mallon E, Watters AD, Cooke T, Paish C, Wencyk PM, Pinder SE (2003) Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol 199: 418–423
Earle CC, Maroun JA (1999) Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer 35: 1059–1064
Gianni L, Panzini I, Tassinari D, Mianulli AM, Desiderio F, Ravaioli A (2001) Meta-analyses of randomized trials of adjuvant chemotherapy in gastric cancer. Ann Oncol 12: 1178–1180
Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, Kim SJ, Markowitz S (2000) Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet 26: 16–17
Green D, Ponce DL, Leon-Rodriguez E, Sosa-Sanchez R (2002) Adenocarcinoma of the stomach: univariate and multivariate analysis of factors associated with survival. Am J Clin Oncol 25: 84–89
Harrison LE, Karpeh MS, Brennan MF (1998) Extended lymphadenectomy is associated with a survival benefit for node-negative gastric cancer. J Gastrointest Surg 2: 126–131
Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ (1993) Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol 11: 1441–1447
Hirashima N, Takahashi W, Yoshii S, Yamane T, Ooi A (2001) Protein overexpression and gene amplification of c-erb B-2 in pulmonary carcinomas: a comparative immunohistochemical and fluorescence in situ hybridization study. Mod Pathol 14: 556–562
Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, Wang L, Wang CH, Chen HY, Li YP (2002) Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol 8: 1023–1028
Janunger KG, Hafstrom L, Glimelius B (2002) Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg 168: 597–608
Janunger KG, Hafstrom L, Nygren P, Glimelius B (2001) A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol 40: 309–326
Kim MA, Jung EJ, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH (2007) Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum Pathol 38: 1386–1393
Kimura M, Tsuda H, Morita D, Ichikura T, Ogata S, Aida S, Yoshizumi Y, Maehara T, Mochizuki H, Matsubara O (2004) A proposal for diagnostically meaningful criteria to classify increased epidermal growth factor receptor and c-erbB-2 gene copy numbers in gastric carcinoma, based on correlation of fluorescence in situ hybridization and immunohistochemical measurements. Virchows Arch 445: 255–262
Luebke T, Baldus SE, Grass G, Bollschweiler E, Thiele J, Dienes HP, Hoelscher AH, Moenig SP (2005) Histological grading in gastric cancer by Ming classification: correlation with histopathological subtypes, metastasis, and prognosis. World J Surg 29: 1422–1427
Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V (2000) Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente). Ann Oncol 11: 837–843
Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, Leiberman G, Slamon DJ (2005) Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer 6: 240–246
McKenzie SJ, DeSombre KA, Bast BS, Hollis DR, Whitaker RS, Berchuck A, Boyer CM, Bast Jr RC (1993) Serum levels of HER-2 neu (C-erbB-2) correlate with overexpression of p185neu in human ovarian cancer. Cancer 71: 3942–3946
Ming SC (1977) Gastric carcinoma. A pathobiological classification. Cancer 39: 2475–2485
Msika S, Benhamiche AM, Jouve JL, Rat P, Faivre J (2000) Prognostic factors after curative resection for gastric cancer. A population-based study. Eur J Cancer 36: 390–396
Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H (1999) The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 85: 1894–1902
Ooi A, Kobayashi M, Mai M, Nakanishi I (1998) Amplification of c-erbB2 in gastric cancer: detection in formalin-fixed, paraffin-embedded tissue by fluorescence in situ hybridization. Lab Invest 78: 345–351
Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH, Son BH, Cho EY, Chae SW, Kim EJ, Sohn JH, Ryu SH, Sepulveda AR (2006) HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 51: 1371–1379
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ (2000) Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 18: 3651–3664
Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D (1999) Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18: 2241–2251
Pegram MD, Finn RS, Arzoo K, Beryt M, Pietras RJ, Slamon DJ (1997) The effect of HER-2/neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene 15: 537–547
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353: 1659–1672
Popescu NC, King CR, Kraus MH (1989) Localization of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12-21.32. Genomics 4: 362–366
Rebischung C, Barnoud R, Stefani L, Faucheron JL, Mousseau M (2005) The effectiveness of trastuzumab (herceptin) combined with chemotherapy for gastric carcinoma with overexpression of the c-erbB-2 protein. Gastric Cancer 8: 249–252
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer Jr CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353: 1673–1684
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792
Takehana T, Kunitomo K, Kono K, Kitahara F, Iizuka H, Matsumoto Y, Fujino MA, Ooi A (2002) Status of c-erbB-2 in gastric adenocarcinoma: a comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int J Cancer 98: 833–837
Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, Elenius K, Isola J (2005) Amplification of HER-2 in gastric carcinoma: association with topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 16: 273–278
Varis A, Zaika A, Puolakkainen P, Nagy B, Madrigal I, Kokkola A, Vayrynen A, Karkkainen P, Moskaluk C, El Rifai W, Knuutila S (2004) Coamplified and overexpressed genes at ERBB2 locus in gastric cancer. Int J Cancer 109: 548–553
Wolf-Yadlin A, Kumar N, Zhang Y, Hautaniemi S, Zaman M, Kim HD, Grantcharova V, Lauffenburger DA, White FM (2006) Effects of HER2 overexpression on cell signaling networks governing proliferation and migration. Mol Syst Biol 2: 54
Xu YH, Richert N, Ito S, Merlino GT, Pastan I (1984) Characterization of epidermal growth factor receptor gene expression in malignant and normal human cell lines. Proc Natl Acad Sci USA 81: 7308–7312
Acknowledgements
This study was supported by Programa Saúde XXI and by a research grant from Liga Portuguesa Contra o Cancro – Núcleo Regional do Norte.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Barros-Silva, J., Leitão, D., Afonso, L. et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer 100, 487–493 (2009). https://doi.org/10.1038/sj.bjc.6604885
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604885
Keywords
This article is cited by
-
The effect of specimen processing time on HER2 expression in gastric cancer and esophagogastric junction cancer: a single-center retrospective observational study
BMC Cancer (2023)
-
Clinical significance of CD166 and HER-2 in different types of gastric cancer
Clinical and Translational Oncology (2023)
-
Human epidermal growth factor 2 overexpressed alpha-fetoprotein-producing-gastric cancer
Discover Oncology (2023)
-
Deviating HER2 test results in gastric cancer: analysis from the prospective multicenter VARIANZ study
Journal of Cancer Research and Clinical Oncology (2023)
-
Prognostic and clinical significance of HER-2 low expression in early-stage gastric cancer
BMC Cancer (2022)