Abstract
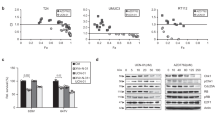
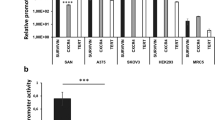
There is a need to enhance the efficacy of genetic prodrug activation therapy using herpes simplex virus thymidine kinase (tk) and ganciclovir (GCV) following disappointing results in early clinical trials. tk/GCV has been shown to lead to the activation of caspase-3, a potent executor of apoptosis. We demonstrate that co-expression of pro-caspase-3 with tk/GCV leads to enhanced cell death in ovarian carcinoma cells in vitro. Following transfection with recombinant adenoviral vectors encoding tk, GCV treatment leads to greater cell death in pro-caspase-3–expressing clones of SKOV3 and IGROV1 than control cells, as well as more rapid activation of caspase-3 and more rapid cleavage of PARP. Flow cytometry suggests that there is a greater degree of S-phase block in the pro-caspase-3–expressing clones than in control cells following treatment with tk/GCV. None of these effects is seen following transfection with a control adenovirus that does not encode tk. The increased cell death, early caspase-3 activation and PARP cleavage, and flow cytometric changes seen in pro-caspase-3–expressing cells can be partially inhibited by treatment with benzyloxycarbonyl–val–ala–asp fluoromethylketone, a synthetic caspase inhibitor. Our data suggest that co-expression of pro-caspase-3 may lead to a significant enhancement of the efficacy of tk/GCV therapy. Cancer Gene Therapy (2001) 8, 308–319
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Huber B, Austin E, Richards C, et al . Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene — significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase Proc Natl Acad Sci USA 1994; 91:: 8302–8306
McNeish I, Green N, Gilligan M, et al . Virus-directed enzyme prodrug therapy for ovarian and pancreatic cancer using retrovirally delivered E. coli nitroreductase and CB1954 Gene Ther 1998; 5:: 1061–1069
Rainov N, Dobberstein K, Sena-Esteves M, et al . New prodrug activation gene therapy for cancer using cytochrome P450 4B1 and 2-aminoanthracene/4-ipomeanol Hum Gene Ther 1998; 9:: 1261–1273
Cortés M, de Felipe P, Martin V, et al . Successful use of a plant gene in the treatment of cancer in vivo Gene Ther 1998; 5:: 1499–1507
Kojima A, Hackett N, Ohwada A, et al . In vivo human carboxylesterase cDNA gene transfer to activate the prodrug CPT-11 for local treatment of solid tumors J Clin Invest 1998; 101:: 1789–1796
Moolten F . Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy Cancer Res 1986; 46:: 5276–5281
Izquierdo M, Martin V, de Felipe P, et al . Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy Gene Ther 1996; 3:: 491–495
Ram Z, Culver K, Oshiro E, et al . Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producer cells Nat Med 1997; 4:: 1354–1361
Shand N, Weber F, Mariani L, et al . A phase 1–2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir Hum Gene Ther 1999; 10:: 2325–2335
Klatzmann D, Valery C, Bensimon G, et al . A phase I/II study of herpes simplex virus type I thymidine kinase “suicide” gene therapy for recurrent glioblastoma Hum Gene Ther 1998; 9:: 2595–2604
Herman J, Adler H, Aguilar-Cordova E, et al . In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial Hum Gene Ther 1999; 10:: 1239–1249
Loubiere L, Tiraby M, Cazaux C, et al . The equine herpes virus 4 thymidine kinase leads to a superior ganciclovir cell killing than the human herpes virus 1 thymidine kinase Gene Ther 1999; 6:: 1638–1642
Cannon J, Hamzeh F, Moore S, et al . Human herpes virus 8–encoded thymidine kinase and phosphotransferase homologues confer sensitivity of ganciclovir J Virol 1999; 73:: 4786–4793
Kokoris M, Sabo P, Adman E, et al . Enhancement of tumor ablation by a selected HSV-1 thymidine kinase mutant Gene Ther 1999; 6:: 1415–1426
Tong X, Engehausen D, Kaufman R, et al . Improvement of gene therapy for ovarian cancer by using acyclovir instead of ganciclovir in adenovirus-mediated thymidine kinase gene therapy Anticancer Res 1998; 18:: 713–718
Uckert W, Kammertons T, Haack K, et al . Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo Hum Gene Ther 1998; 9:: 855–865
Chen S, Chen X, Wang Y, et al . Combination gene therapy for liver metastasis of colon carcinoma in vivo Proc Natl Acad Sci USA 1995; 92:: 2577–2581
Benedetti S, Dimeco F, Pollo B, et al . Limited efficacy of the HSV-TK/GCV system for gene therapy of malignant gliomas and perspectives for the combined transduction of the interleukin-4 gene Hum Gene Ther 1997; 8:: 1345–1353
Wei S-J, Chao Y, Shih Y-L, et al . Involvement of Fas (CD95/APO-1) and Fas ligand in apoptosis induced by ganciclovir treatment of tumor cells transduced with herpes simplex virus thymidine kinase Gene Ther 1999; 6:: 420–431
Beltinger C, Fulda S, Kammertoens T, et al . Herpes simplex virus thymidine kinase/ganciclovir–induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases Proc Natl Acad Sci USA 1999; 96:: 8699–8704
Nicholson D . Caspase structure, proteolytic substrates, and function during apoptotic cell death Cell Death Differ 1999; 6:: 1028–1042
Shinoura N, Murumatsa Y, Yoshida Y, et al . Adenovirus-mediated transfer of caspase-3 with Fas ligand induces drastic apoptosis in U-373MG glioma cells Exp Cell Res 2000; 256:: 423–433
Yamabe K, Shimizu S, Ito T, et al . Cancer gene therapy using a pro-apoptotic gene, caspase-3 Gene Ther 1999; 6: 1952–1959
Srinivasula S, Ahmad M, MacFarlane M, et al . Generation of constitutively active recombinant caspases-3 and -6 by rearrangement of their subunits J Biol Chem 1998; 273:: 10107–10111
Tenev T, Marani M, McNeish I, et al . Caspase-3 overexpression sensitizes ovarian cancer cells to proteasome inhibitors Cell Death Differ 2000 In press
Garcia-Calvo M, Peterson E, Leiting B, et al . Inhibition of human caspases by peptide-based and macromolecular inhibitors J Biol Chem 1998; 273:: 32608–32613
Tenev T, Böhmer S-A, Kaufmann R, et al . Perinuclear localization of the protein-tyrosine phosphatase SHP-1 and inhibition of epidermal growth factor–stimulated STAT1/3 activation in A431 cells Eur J Cell Biol 2000; 79:: 261–271
Vassaux G, Hurst H, Lemoine N . Insulation of a conditionally expressed transgene in an adenoviral vector Gene Ther 1999; 6:: 1192–1197
Ring C, Blouin P, Martin L-A, et al . Use of transcriptional regulatory elements of the MUC1 and ERBB2 genes to drive tumor-selective expression of a prodrug activating enzyme Gene Ther 1997; 4:: 1045–1052
Mosmann T . Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays J Immunol Methods 1983; 65:: 55–63
Yu D, Wolf J, Scanlon M, et al . Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A Cancer Res 1993; 53:: 891–898
Kovarik A, Peat N, Wilson D, et al . Analysis of the tissue-specific promoter of the MUC1 gene J Biol Chem 1993; 268:: 9917–9926
Lazebnik Y, Kaufmann S, Desnoyers S, et al . Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE Nature 1994; 371:: 346–347
Nicholson DW, Ali A, Thornberry NA, et al . Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis Nature 1995; 376:: 37–43
Decker T, Lohmannmatthes ML . A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity J Immunol Methods 1988; 115:: 61–69
Jänicke R, Ng P, Sprengart M, et al . Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis J Biol Chem 1998; 273:: 15540–15545
Clarke R, Lund E, Johnson I, et al . Apoptosis can be detected in attached colonic adenocarcinoma HT29 cells using annexin V binding, but not by TUNEL assay or sub G0 DNA content Cytometry 2000; 39:: 141–150
Melcher A, Todryk S, Hardwick S, et al . Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression Nat Med 1998; 4:: 581–587
Laster S, Wood J, Gooding L . Tumor necrosis factor can induce both apoptotic and necrotic forms of cell lysis J Immunol 1988; 141:: 2629–2635
Saldeen J . Cytokines induce both necrosis and apoptosis via a common bcl-2 inhibitable pathway in rat insulin-producing cells Endocrinology 2000; 141:: 2003–2010
Vercammen D, Brouckaert G, Denecker G, et al . Dual signalling of the Fas receptor: initiation of both apoptotic and necrotic cell death receptors J Exp Med 1998; 188: 919–930
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McNeish, I., Tenev, T., Bell, S. et al. Herpes simplex virus thymidine kinase/ganciclovir–induced cell death is enhanced by co-expression of caspase-3 in ovarian carcinoma cells. Cancer Gene Ther 8, 308–319 (2001). https://doi.org/10.1038/sj.cgt.7700305
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700305
Keywords
This article is cited by
-
Tumoricidal effect of human olfactory ensheathing cell mediated suicide gene therapy in human glioblastoma cells
Molecular Biology Reports (2018)
-
Failure of Translation of Human Adenovirus mRNA in Murine Cancer Cells Can be Partially Overcome by L4-100K Expression In Vitro and In Vivo
Molecular Therapy (2012)
-
Inhibition of the Inflammatory Cytokine TNF-α Increases Adenovirus Activity in Ovarian Cancer via Modulation of cIAP1/2 Expression
Molecular Therapy (2011)
-
Oncolytic adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer
Oncogene (2008)
-
Transcellular transfer of active HSV-1 thymidine kinase mediated by an 11-amino-acid peptide from HIV-1 Tat
Cancer Gene Therapy (2003)