-
PDF
- Split View
-
Views
-
Cite
Cite
Johannes Sarnthein, Jair Stern, Christoph Aufenberg, Valentin Rousson, Daniel Jeanmonod, Increased EEG power and slowed dominant frequency in patients with neurogenic pain, Brain, Volume 129, Issue 1, January 2006, Pages 55–64, https://doi.org/10.1093/brain/awh631
Close - Share Icon Share
Abstract
To study the mechanisms of chronic neurogenic pain, we compared the power spectra of the resting EEG of patients (n = 15, 38–75 years, median 64 years, 6 women) and healthy controls (n = 15, 41–71 years, median 60 years, 8 women). On an average, the patient group exhibited higher spectral power over the frequency range of 2–25 Hz, and the dominant peak was shifted towards lower frequencies. Maximal differences appeared in the 7–9 Hz band in all electrodes. Frontal electrodes contributed most to this difference in the 13–15 Hz band. Bicoherence analysis suggests an enhanced coupling between theta (4–9 Hz) and beta (12–25 Hz) frequencies in patients. The subgroup of six patients free from centrally acting medication showed higher spectral power in the 2–18 Hz frequency range. On an individual basis, the combination of peak height and peak frequency discriminated between patient and control groups: discriminant analysis classified 87% of all subjects correctly. After a therapeutic lesion in the thalamus (central lateral thalamotomy, CLT) we carried out follow-up for a subgroup of seven patients. Median pain relief was 70 and 95% after 3 and 12 months, respectively. The average EEG power of all seven patients gradually decreased in the theta band and approached normal values only after 12 months. The excess theta EEG power in patients and its decrease after thalamic surgery suggests that both EEG and neurogenic pain are determined by tightly coupled thalamocortical loops. The small therapeutic CLT lesion is thought to initiate a progressive normalization in the affected thalamocortical system, which is reflected in both decrease of EEG power and pain relief.
Introduction
While several mechanisms have been proposed for the pathophysiology of neurogenic pain (Treede et al., 1999; Peyron et al., 2000; Jones et al., 2003; Apkarian et al., 2005) no general agreement has been reached yet. In the following we refer to a mechanism that focuses on thalamocortical interplay (Llinás et al., 1999). First evidence for this mechanism was the finding of low-threshold calcium spike (LTS) bursts in the somatosensory thalamus of patients with neurogenic pain (Lenz et al., 1989). A detailed investigation (Jeanmonod et al., 1993) in the medial thalamus (central lateral nucleus, CL) showed that (i) half of all recorded neurons presented LTS bursting activity, (ii) only a minority (<1%) had somatosensory receptive fields and (iii) LTS bursts displayed a theta rhythmicity, with a mean interburst discharge rate of 4 Hz. LTSs have been described intracellularly in in vitro and in vivo experiments and have been related to a state of membrane hyperpolarization (Llinás and Jahnsen, 1982; Steriade, 2001). CL is part of the medial thalamus, is diffusely connected to wide cortical areas (Steriade et al., 1997; Jones, 2001) and is thought to serve as the non-specific amplifier of thalamocortical activity (Llinas et al., 2002). Functionally, the tight coupling of the thalamocortical re-entry loop is reflected by high thalamocortical coherence in human patients (Sarnthein et al., 2003). This loop constitutes an important component contributing to the rhythmicity of scalp EEG and magnetoencephalography (MEG) (Nunez et al., 2001). Furthermore, slowed EEG/MEG rhythmicity has been reported in a few patients with neurogenic pain (Gücer et al., 1978; Llinás et al., 1999; Sarnthein et al., 2003). Based on these physiological findings and the clinical fact that a therapeutic lesion in CL (central lateral thalamotomy, CLT) relieves pain and other functional brain disorders (Jeanmonod et al., 1993, 1996, 2001a), thalamocortical dysrhythmia (TCD) was proposed as a general mechanism to explain the generation of neurogenic pain and other positive neurological symptoms (Llinás et al., 1999, 2001; Jeanmonod et al., 2001b). However, the pioneering reports of slowed EEG/MEG rhythmicity in neurogenic pain were related to only a small number of patients.
The present study aimed, first, to discriminate statistically between neurogenic pain patients and healthy controls on the basis of scalp EEG spectral parameters. Second, we hypothesized a theta reduction in patients' EEG after the surgical intervention CLT, which has the goal to reduce thalamic LTS production. We therefore monitored the EEG 3 and 12 months after the therapeutic lesion.
Methods
Patients
The patient group consisted of 17 patients with severe forms of neurogenic pain that fulfilled all admission criteria for CLT neurosurgical therapy (see below). Following the terminology of the International Association for the Study of Pain, we use the term neurogenic pain to refer to the pain initiated or caused by a primary lesion, dysfunction, or transitory perturbation in the peripheral or central nervous system. Of the initial patient group, we excluded one patient due to eye movement artefacts in the EEG and one patient because of low voltage EEG. Symptoms, medication and pain relief reported by the remaining patients in the group (n = 15, 38–75 years, median 64 years, 6 women, 9 men) are listed in Table 1. After surgery, three patients died due to unrelated reasons, two were not available for EEG and three await EEG follow-up to date. Therefore, a subgroup of n = 7 patients was available for recording EEG at 3 and 12 months after surgery.
Clinical description of individual patients
| Patient . | . | . | . | . | . | . | Preoperative . | . | Surgery target . | 3 months . | . | 12 months . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age . | Sex . | Pain location . | Pain side . | Pain type/cause . | Lesion level . | VAS . | Drugs (%) . | . | Relief . | Drugs (%) . | Relief . | Drugs (%) . | |||||||||
| 1 | 75 | F | Trigeminal | Right | co,al,ep/herpes | p | 75 | BZ | CLT lr | |||||||||||||
| 2 | 68 | M | Trigeminal | Right | co,al,ep,pa/tumour | p | 60 | – | CLT lr | 100 | – | 100 | – | |||||||||
| 3 | 38 | M | Trigeminal | Right | al,ep,pa/tumour | p | 70 | AE, AD | CLT ri | 80 | >AE | 95 | – | |||||||||
| 4 | 51 | F | Trigeminal | Right | co,al,ep,pa/idiopathic | p | 50 | BZ, AD, OP | CLT le | 50 | BZ, AD, >OP | 95 | >BZ, AD | |||||||||
| 5 | 66 | M | Trigeminal | Right | al,pa/vascular | c | 100 | AE, OP | CLT ri | 100 | >AE, BZ | 100 | – | |||||||||
| 6 | 69 | F | Trigeminal | Right | co,al,pa/idiopathic | p | 75 | BZ, AD | CLT ri | |||||||||||||
| 7 | 73 | M | Trigeminal | Left | co,pa/tumour | c | 63 | BZ, AD | CLT le | |||||||||||||
| 8 | 62 | M | Leg | Right | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 9 | 58 | F | Leg | Left | co,ep/root lesion | p | 73 | AE, BZ, AD, OP | CLT ri | 25 | >AE,BZ, AD,OP | 95 | >BZ, AD | |||||||||
| 10 | 54 | F | Leg | Left | co/root lesion | p | 65 | – | CLT ri | 70 | – | 75 | – | |||||||||
| 11 | 64 | M | Leg | Left | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 12 | 68 | M | Leg | Left | co,al,ep/spinal cord lesion | c | 80 | – | CLT ri | |||||||||||||
| 13 | 73 | F | Hemibody | Left | co/vascular | c | 65 | – | CLT ri | |||||||||||||
| 14 | 52 | M | Cervical | Right | co,ep/tumour/neurectomy | p | 60 | AE, BZ,OP | CLT le | 0 | <AE, AD, >OP | 0 | >AE, BZ | |||||||||
| 15 | 61 | M | Arm | Both | co,al/polyneuropathy | p | 75 | AE, AD, OP | CLT lr | |||||||||||||
| Median pain relief | 70 | 95 | ||||||||||||||||||||
| Patient . | . | . | . | . | . | . | Preoperative . | . | Surgery target . | 3 months . | . | 12 months . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age . | Sex . | Pain location . | Pain side . | Pain type/cause . | Lesion level . | VAS . | Drugs (%) . | . | Relief . | Drugs (%) . | Relief . | Drugs (%) . | |||||||||
| 1 | 75 | F | Trigeminal | Right | co,al,ep/herpes | p | 75 | BZ | CLT lr | |||||||||||||
| 2 | 68 | M | Trigeminal | Right | co,al,ep,pa/tumour | p | 60 | – | CLT lr | 100 | – | 100 | – | |||||||||
| 3 | 38 | M | Trigeminal | Right | al,ep,pa/tumour | p | 70 | AE, AD | CLT ri | 80 | >AE | 95 | – | |||||||||
| 4 | 51 | F | Trigeminal | Right | co,al,ep,pa/idiopathic | p | 50 | BZ, AD, OP | CLT le | 50 | BZ, AD, >OP | 95 | >BZ, AD | |||||||||
| 5 | 66 | M | Trigeminal | Right | al,pa/vascular | c | 100 | AE, OP | CLT ri | 100 | >AE, BZ | 100 | – | |||||||||
| 6 | 69 | F | Trigeminal | Right | co,al,pa/idiopathic | p | 75 | BZ, AD | CLT ri | |||||||||||||
| 7 | 73 | M | Trigeminal | Left | co,pa/tumour | c | 63 | BZ, AD | CLT le | |||||||||||||
| 8 | 62 | M | Leg | Right | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 9 | 58 | F | Leg | Left | co,ep/root lesion | p | 73 | AE, BZ, AD, OP | CLT ri | 25 | >AE,BZ, AD,OP | 95 | >BZ, AD | |||||||||
| 10 | 54 | F | Leg | Left | co/root lesion | p | 65 | – | CLT ri | 70 | – | 75 | – | |||||||||
| 11 | 64 | M | Leg | Left | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 12 | 68 | M | Leg | Left | co,al,ep/spinal cord lesion | c | 80 | – | CLT ri | |||||||||||||
| 13 | 73 | F | Hemibody | Left | co/vascular | c | 65 | – | CLT ri | |||||||||||||
| 14 | 52 | M | Cervical | Right | co,ep/tumour/neurectomy | p | 60 | AE, BZ,OP | CLT le | 0 | <AE, AD, >OP | 0 | >AE, BZ | |||||||||
| 15 | 61 | M | Arm | Both | co,al/polyneuropathy | p | 75 | AE, AD, OP | CLT lr | |||||||||||||
| Median pain relief | 70 | 95 | ||||||||||||||||||||
Pain locations are trigeminal, lower limb (leg), hemibody, cervical, and upper limb (arm). Pain types are constant (co), allodynic (al), episodic (ep) and paroxysmal (pa). Lesion levels are central (c) and peripheral (p). Pain intensity was rated on a visual analogue scale (VAS) in %. Displayed are mean VAS ratings and, in the absence of constant pain, the VAS rating during pain attacks. Relevant drugs include antiepileptics (AE), benzodiazepines (BZ), antidepressants (AD) and opiates (OP). Surgical targets were CLT left (le), CLT right (ri), or CLT left and right (lr). Postoperative development was characterized by decrease (>) or increase (<) in drug dose and percentage of pain relief (%).
Clinical description of individual patients
| Patient . | . | . | . | . | . | . | Preoperative . | . | Surgery target . | 3 months . | . | 12 months . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age . | Sex . | Pain location . | Pain side . | Pain type/cause . | Lesion level . | VAS . | Drugs (%) . | . | Relief . | Drugs (%) . | Relief . | Drugs (%) . | |||||||||
| 1 | 75 | F | Trigeminal | Right | co,al,ep/herpes | p | 75 | BZ | CLT lr | |||||||||||||
| 2 | 68 | M | Trigeminal | Right | co,al,ep,pa/tumour | p | 60 | – | CLT lr | 100 | – | 100 | – | |||||||||
| 3 | 38 | M | Trigeminal | Right | al,ep,pa/tumour | p | 70 | AE, AD | CLT ri | 80 | >AE | 95 | – | |||||||||
| 4 | 51 | F | Trigeminal | Right | co,al,ep,pa/idiopathic | p | 50 | BZ, AD, OP | CLT le | 50 | BZ, AD, >OP | 95 | >BZ, AD | |||||||||
| 5 | 66 | M | Trigeminal | Right | al,pa/vascular | c | 100 | AE, OP | CLT ri | 100 | >AE, BZ | 100 | – | |||||||||
| 6 | 69 | F | Trigeminal | Right | co,al,pa/idiopathic | p | 75 | BZ, AD | CLT ri | |||||||||||||
| 7 | 73 | M | Trigeminal | Left | co,pa/tumour | c | 63 | BZ, AD | CLT le | |||||||||||||
| 8 | 62 | M | Leg | Right | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 9 | 58 | F | Leg | Left | co,ep/root lesion | p | 73 | AE, BZ, AD, OP | CLT ri | 25 | >AE,BZ, AD,OP | 95 | >BZ, AD | |||||||||
| 10 | 54 | F | Leg | Left | co/root lesion | p | 65 | – | CLT ri | 70 | – | 75 | – | |||||||||
| 11 | 64 | M | Leg | Left | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 12 | 68 | M | Leg | Left | co,al,ep/spinal cord lesion | c | 80 | – | CLT ri | |||||||||||||
| 13 | 73 | F | Hemibody | Left | co/vascular | c | 65 | – | CLT ri | |||||||||||||
| 14 | 52 | M | Cervical | Right | co,ep/tumour/neurectomy | p | 60 | AE, BZ,OP | CLT le | 0 | <AE, AD, >OP | 0 | >AE, BZ | |||||||||
| 15 | 61 | M | Arm | Both | co,al/polyneuropathy | p | 75 | AE, AD, OP | CLT lr | |||||||||||||
| Median pain relief | 70 | 95 | ||||||||||||||||||||
| Patient . | . | . | . | . | . | . | Preoperative . | . | Surgery target . | 3 months . | . | 12 months . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age . | Sex . | Pain location . | Pain side . | Pain type/cause . | Lesion level . | VAS . | Drugs (%) . | . | Relief . | Drugs (%) . | Relief . | Drugs (%) . | |||||||||
| 1 | 75 | F | Trigeminal | Right | co,al,ep/herpes | p | 75 | BZ | CLT lr | |||||||||||||
| 2 | 68 | M | Trigeminal | Right | co,al,ep,pa/tumour | p | 60 | – | CLT lr | 100 | – | 100 | – | |||||||||
| 3 | 38 | M | Trigeminal | Right | al,ep,pa/tumour | p | 70 | AE, AD | CLT ri | 80 | >AE | 95 | – | |||||||||
| 4 | 51 | F | Trigeminal | Right | co,al,ep,pa/idiopathic | p | 50 | BZ, AD, OP | CLT le | 50 | BZ, AD, >OP | 95 | >BZ, AD | |||||||||
| 5 | 66 | M | Trigeminal | Right | al,pa/vascular | c | 100 | AE, OP | CLT ri | 100 | >AE, BZ | 100 | – | |||||||||
| 6 | 69 | F | Trigeminal | Right | co,al,pa/idiopathic | p | 75 | BZ, AD | CLT ri | |||||||||||||
| 7 | 73 | M | Trigeminal | Left | co,pa/tumour | c | 63 | BZ, AD | CLT le | |||||||||||||
| 8 | 62 | M | Leg | Right | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 9 | 58 | F | Leg | Left | co,ep/root lesion | p | 73 | AE, BZ, AD, OP | CLT ri | 25 | >AE,BZ, AD,OP | 95 | >BZ, AD | |||||||||
| 10 | 54 | F | Leg | Left | co/root lesion | p | 65 | – | CLT ri | 70 | – | 75 | – | |||||||||
| 11 | 64 | M | Leg | Left | co/trauma | p | 60 | – | CLT le | |||||||||||||
| 12 | 68 | M | Leg | Left | co,al,ep/spinal cord lesion | c | 80 | – | CLT ri | |||||||||||||
| 13 | 73 | F | Hemibody | Left | co/vascular | c | 65 | – | CLT ri | |||||||||||||
| 14 | 52 | M | Cervical | Right | co,ep/tumour/neurectomy | p | 60 | AE, BZ,OP | CLT le | 0 | <AE, AD, >OP | 0 | >AE, BZ | |||||||||
| 15 | 61 | M | Arm | Both | co,al/polyneuropathy | p | 75 | AE, AD, OP | CLT lr | |||||||||||||
| Median pain relief | 70 | 95 | ||||||||||||||||||||
Pain locations are trigeminal, lower limb (leg), hemibody, cervical, and upper limb (arm). Pain types are constant (co), allodynic (al), episodic (ep) and paroxysmal (pa). Lesion levels are central (c) and peripheral (p). Pain intensity was rated on a visual analogue scale (VAS) in %. Displayed are mean VAS ratings and, in the absence of constant pain, the VAS rating during pain attacks. Relevant drugs include antiepileptics (AE), benzodiazepines (BZ), antidepressants (AD) and opiates (OP). Surgical targets were CLT left (le), CLT right (ri), or CLT left and right (lr). Postoperative development was characterized by decrease (>) or increase (<) in drug dose and percentage of pain relief (%).
Surgery
Neurosurgical therapy for the patients consisted of CLT, a therapeutic lesion in the posterior part of the thalamic nucleus, CL (Morel et al., 1997), and has been described in detail previously (Jeanmonod et al., 2001a). Admission criteria for CLT include resistance to drug therapies (antiepileptics, benzodiazepines and antidepressants), chronic pain state >1 year, severe suffering and a strongly diminished quality of life. Distinct from all other lesional procedures applied in chronic pain, CLT targets cells in the posterior part of CL which have been found to be functionally blocked. Therefore the normal functions of CL are spared by CLT, since they have been transferred to other areas by plastic reorganization of the thalamocortical network. In a previous study with n = 96 patients and a follow-up of 4 years, >50% of the patients obtained pain relief (satisfactory up to complete relief) (Jeanmonod et al., 2001a).
Healthy controls
The healthy control group consisted of 15 subjects (41–71 years, median 60 years, 8 women, 7 men). The control subjects had no current or previous history of relevant physical illness and they were not currently taking drugs or medication known to affect their EEG. The healthy, age-matched control group was selected to statistically delineate the EEG abnormality reported in a small number of patients (Gücer et al., 1978; Llinás et al., 1999; Sarnthein et al., 2003). Furthermore, the healthy control group served as a reference when postoperative changes in the patients EEG were monitored.
EEG recording sessions
The study was approved by the Kanton Zürich ethics committee. All subjects, patients and controls, were informed about the aim and the scope of the study and all gave written informed consent according to the Declaration of Helsinki. Subjects were seated in a dimly lit room shielded against sound and stray electric fields and were video-monitored. All EEGs were acquired in the morning between 9 and 12 h. Recording sessions of patients and controls were followed by an interleaved schedule and the recording apparatus was continuously calibrated. Subjects refrained from caffeinated beverages before the session to avoid the caffeine-induced theta decrease in EEG (Landolt et al., 2004). Since drowsiness may result in enhanced theta power, the vigilance of subjects was checked. In addition, patients were routinely asked whether they had sleeping disorders because insomnia conflicts with the typical clinical diagnosis of neurogenic pain.
Within each session, spontaneous EEG was recorded under two conditions: while subjects rested with their eyes closed, and while they rested with their eyes open. EEG was recorded for 5 min under each condition. We focused our analysis on the eyes closed condition as it is less prone to artefacts and we assume that an internal process like neurogenic pain should be more easily accessed in the brain's ‘idling mode’ (Pfurtscheller et al., 1996), unmasked by sensory perception. Therefore all results presented in this study refer to the eyes closed condition, except for Fig. 1D. We refrained from provoking acute pain since we are interested in the pathophysiology of chronic neurogenic pain that is experienced independent of nociceptive stimuli. Before each recording segment, subjects were instructed to assume a comfortable position in a chair. They were free to place their head on a chin-rest. For the eyes closed condition, subjects were instructed to close their eyes, to place their fingers on their eyelids, and to relax but to stay awake. After 5 min subjects were instructed to open their eyes, to fixate on a dot at 1 m distance and to relax.
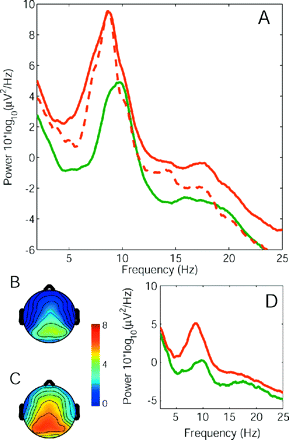
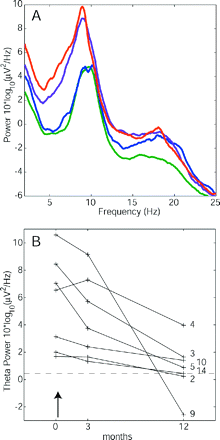
Average power spectra. The spectrum for the group of patients (red) and the group of healthy controls (green) with eyes closed (A). The global EEG power was enhanced in patients and the dominant peak was shifted towards lower frequencies in the patient population. The subgroup of six patients free from centrally acting medication showed the same effect in the 2–18 Hz frequency range (A, dashed line). The topographical distribution of EEG power (5–13 Hz, eyes closed) on the scalp of control group (B) and patient group (C) had a maximum at parietal electrode sites, but extended to more frontal regions in the patient group. (D) Upon opening the eyes, the power spectral density was reduced in both the groups, but theta overactivity persisted in the patient group.
EEG signals were measured using 60 Ag/AgCl surface electrodes, which were fixed in a cap at the standard positions according to the extended 10–20 system (FMS11, Falk Minow Services, Herrsching, Germany). Electrode CPz served as the common reference. Impedances were below 5 kΩ in all electrodes processed in the further analysis. We used two additional bipolar electrode channels as eye monitors. EEG signals were registered using the SynAmps EEG system (Neuroscan Compumedics, Houston, TX, 16 bit A/D conversion, sampling rate 250 Hz, 0.3–100 Hz band pass filter, −12 dB/octave) and continuously viewed on a PC monitor.
Data preprocessing and editing
Data were analysed offline using Matlab (The Mathworks, Natick, MA) using EEGLAB (http://sccn.ucsd.edu/eeglab; Delorme and Makeig, 2004) and custom scripts. The scalp EEG was re-referenced to the mean of the signals recorded at the ear lobes. We confirmed alertness of subjects during the recording session by checking for slowing of the alpha rhythm, slow rolling eye movements or increasing theta power (4–9 Hz). Data were inspected in 5 s epochs, and large muscle or eye movement artefacts were removed. For editing purposes, muscle artefact was considered significant if the underlying EEG rhythms were not clearly seen. The EEG was decomposed into independent components using blind separation (independent component analysis). After the removal of components containing eye movement or muscle artefacts, the signal was reconstructed. This procedure resulted in >60 s of EEG for estimates of power spectral density (mean 244 ± 50 s). For the analysis of inter-frequency relationships, a stretch of >20 s EEG was selected where power spectra remained unchanged over time (mean 150 ± 86 s). All records were edited using the same encephalographer in order to increase reliability.
Data analysis
For power spectral density estimates, the multitaper FFT method was applied to 5 s windows with K = 3 tapers and a bandwidth parameter 2W = 0.8 Hz, leading to a time bandwidth product 2WT = 4 (Percival and Walden, 1993). In the comparisons between spectral parameters, all P values are two-sided from non-parametric Wilcoxon tests. Wherever necessary, EEG spectra were subdivided into frequency bands theta (4–9 Hz), alpha (9–12 Hz), beta (12–25 Hz) and gamma (25–100 Hz). In order to summarize the data and because spectra from all electrodes had similar shape and scale, we averaged the log-transformed spectra of all scalp electrodes for each subject if not stated otherwise.
In order to classify individual subjects into patients and controls on the basis of EEG parameters, classical linear discriminant analyses were carried out. That is, we were seeking for the linear combination of the parameters that best separated the two groups in the sense that it maximized the ‘between-to-within’ variance ratio. Each subject was then assigned to the closest group based on this linear combination. For this step, cross-validation has been used (SPSS version 11.5) such that the chance level of assigning a subject to the correct group was 50%. Exact 95% confidence intervals for the true proportions of correct classification using EEG parameters were calculated from tabulated values for the binomial distribution and we could check whether the chance level 50% was within or outside these confidence intervals.
To learn about the relationship between power at two frequencies f1 and f2 in one EEG signal, we computed bicoherence for each electrode separately. Bicoherence estimates the second-order phase coupling by normalizing the bispectrum B(f1, f2) = 〈X(f1) X(f2) X*(f1 + f2)〉 such that bicoherence is confined to [0 1], where X(f1) is the complex Fourier transform of the signal at frequency f1 (Schack et al., 2002). Bicoherence provides phase information as additional information beyond the power spectrum.
Results
Slowed EEG rhythmicity
As a first result, Fig. 1A shows a clear difference between the average power spectra in the resting EEG of the patient group and the healthy control group. In the patient group, spectral power was higher than in the control group over the whole frequency range (2–25 Hz), and the dominant peak was shifted towards lower frequencies. The subgroup of six patients free from centrally acting medication showed the same effect in the 2–18 Hz frequency range (Fig. 1A, dashed line). The scalp topographical distribution of EEG power at the dominant frequency was maximal in posterior electrodes (Fig. 1B and C). This was expected since subjects had their eyes closed (Berger, 1930; Nunez et al., 2001). In the patient group the region of high power extended to more fronto-central electrodes (Fig. 1C). In both patient and control groups, the dominant peak of the EEG was reduced (‘blocked’) when subjects opened their eyes (Fig. 1D) thus displaying a well-established characteristic of the classical alpha rhythm (Berger, 1930; Pfurtscheller et al., 1996; Nunez et al., 2001; Sarnthein et al., 2005). Nevertheless, also with eyes open the theta overactivity persisted in the patient group.
Individual patients
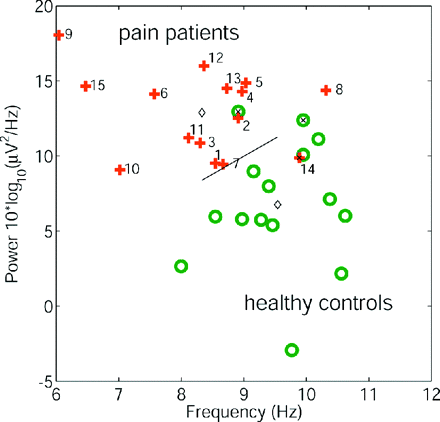
For each subject the height and the frequency of the dominant peak are plotted in Fig. 2. Patients and controls are given as two separate groups. The power values at the dominant frequency come from two distributions with significantly different medians (patients 13.7 × 10 * log10(μ2/Hz), controls 6.2 × 10 * log10(μ2/Hz), P < 0.0001, two-tailed Wilcoxon rank sum test). Median dominant frequencies were 8.6 and 9.4 Hz for the patient group and the control group, respectively (P < 0.002). Discriminant analysis classified 73% of all subjects correctly based only on peak frequency (95% CI 54–88%), 80% based only on peak height (61–92%) and 87% (69–96%) when both the parameters were accounted for. Since cross-validation was used to estimate the classification rate and all confidence intervals exclude the 50% level (chance level), our results are significant at the 95% level.
Individual subject EEG spectral parameters. Height and frequency of the dominant peak for each subject in the patient group (crosses) and the control group (circles). Numbers denote respective patients (Table 1). Using the parameters peak height and peak frequency, bivariate discriminant analysis (group centres: diamonds, misclassified subjects: ‘x’) classified 87% of all subjects correctly.
Medication and clinical data of the patients are listed in Table 1. Of the 15 patients, 9 took medication known to be centrally acting and which could have affected their EEG (Niedermeyer and Lopes da Silva, 1999; Ebersole and Pedley, 2003). For example, those patients (9, 15) with very high and very slow dominant peak took antiepileptic drugs (AE, Table 1). This is in line with the known effect of AE on EEG, which, however, displays a large inter-individual variability (Salinsky et al., 2003). There are thus two cumulative components, AE effects and neurogenic pain pathophysiology, both of which result in a slowed rhythmicity of the EEG of our patients. Effects of other drugs like antidepressants and opiates could not consistently be related to EEG parameters of individual patients. The effect of neurogenic pain pathophysiology alone is documented by the subgroup of six patients without centrally acting medication (NM). On one hand, the enhanced beta power above 18 Hz seen between the solid red curve (all patients) and the dashed red curve (NM subgroup) in Fig. 1A is compatible with the influence of benzodiazepines (Lindhardt et al., 2001) and may illustrate the additive effect of medication and neurogenic pain. On the other hand, the EEG peak parameters of the NM subgroup were well distinct from the healthy control group and the discriminant analysis classified 86% (64–97%) of all subjects correctly.
Topography and frequency dependence
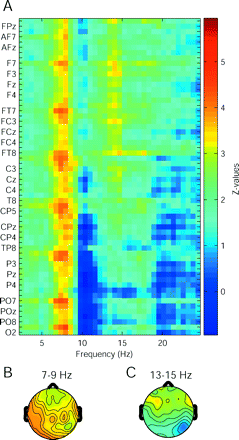
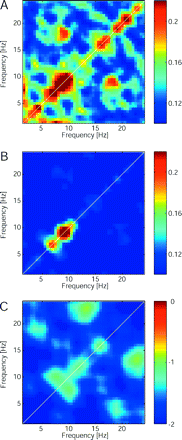
After having established a significant difference between patient group and control group in spectra averaged over all electrodes, we were interested to know which electrodes contributed most to this difference and at what frequency. We performed Wilcoxon rank sum tests for each electrode at each frequency point and plotted the matrix of Z-values as given in Fig. 3A. Maximal Z-values appeared in the 7–9 Hz band in all the electrodes, leading to a rather flat topography (Fig. 3B). Owing to the alpha peak of healthy controls of ∼10 Hz, Z-values were negative in parietal and occipital electrodes (deep blue area in Fig. 3A). In the 13–15 Hz band, Z-values were high in frontal electrodes (Fig. 3C).
Comparison of medians of the patient and control groups. (A) Shown are Z-values for each electrode and frequency point (Wilcoxon rank sum tests). (B) High Z-values occurred from 7–9 Hz in all electrodes. (C) Between 13 and 15 Hz, Z-values were maximal in frontal electrodes.
Interfrequency relationships
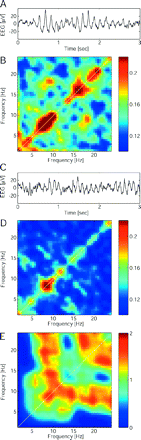
We next investigated the relationship between different frequencies in the EEG. In patients we found bicoherence maxima in the theta and beta bands at the fronto-central electrode FCz, indicating phase correlations of oscillatory events in these frequency bands with their first harmonic (Fig. 4). Further maxima indicate that phase coupling also occurred between theta and beta frequencies. In healthy controls, less interfrequency relationships were visible (Fig. 4D). In part, the coupling between an oscillation and its overtone can be explained by the triangular shape of the EEG waves at the dominant frequency f. The triangular waveshape is visible in the raw EEG (Fig. 4A) and can lead to a peak at the second harmonic at 2 * f in the power spectrum (Dumermuth et al., 1971). The waveshape also explains why power covaries at the fundamental and the overtone frequencies. Patients exhibit higher theta–beta bicoherence at FCz, because the alpha-shaped waves at their dominant frequency extend to more frontal electrodes. Of course triangular waves are common in the EEG of healthy subjects and in themselves not pathological (Niedermeyer and Lopes da Silva, 1999). Only the enhanced occurrence of triangular waves suggests an abnormal coupling between theta and beta frequencies in patients.
Inter-frequency relationships for the EEG signal recorded at the electrode FCz. (A) The raw EEG waveform of patient 10 is asymmetric in the sense that positive peaks are sharper than negative peaks. This triangular waveshape resembles the function sin2(x) and results in enhanced second-order phase coupling between the theta band oscillation and its overtone in the beta band for this patient. (B) Bicoherence averaged over the patient group shows second-order phase coupling in the diagonal and also between theta and beta bands. (C) In the raw EEG waveform of a healthy control, the asymmetry between positive and negative peaks is less pronounced. (D) Average bicoherence in the healthy control group lacks off-diagonal peaks. (E) Z-values for the comparison between bicoherence maps of the patient group and the healthy control group (Wilcoxon rank sum tests). Bicoherence tends to be higher in patients in the diagonal as well as for theta–beta coupling.
Postoperative development
Finally, we were interested in the effect of the therapeutic CLT lesion on the EEG. A subgroup of n = 7 patients was available for EEG recording at 3 and 12 months postoperatively. Of these, one patient reported 0% pain relief and six patients reported immediate or gradually increasing pain relief (median 95%, Table 1). The average EEG spectrum of this patient subgroup gradually approached the average spectrum of the healthy control group (Fig. 5A). In all the patients we also found a gradual decrease in theta power towards the level of the healthy control group (Fig. 5B). Comparison of bicoherence patterns before and 1 year after the surgery showed a reduction of inter-frequency coupling (Fig. 6).
Reduction of EEG power after the therapeutic lesion CLT. (A) The average spectrum of a patient subgroup (n = 7) before surgery (red), after 3 months (violet) and after 12 months (blue) gradually approached that of the healthy control group (green). (B) The power level in the theta band (4–9 Hz) is plotted before (months = 0) and after surgery (↑). After 12 postoperative months, theta reduction was observed in all the seven patients (P < 0.02). Patient theta levels approached the average of the healthy control group (dashed line).
Reduction of EEG bicoherence at the electrode FCz after the therapeutic lesion CLT. (A) Bicoherence values averaged over the patient subgroup (n = 7) before surgery. (B) One year after the surgery, off-diagonal peaks were diminished. (C) The Z-values for the paired comparison between bicoherence maps (Wilcoxon sign rank tests) show a general decrease of interfrequency coupling in the patient group 1 year after surgery.
While a postoperative change in theta power could in principle also arise from normal test–retest variability of the EEG (Salinsky et al., 1991), in our group (n = 7) the theta reduction preoperative versus 12 months postoperative is probably related to the surgical intervention (P < 0.02, paired Wilcoxon sign rank test). In other frequency bands we observed only insignificant changes. While in three patients (3, 5, 9) the reduction of AE could have contributed to theta reduction (Salinsky et al., 2003), also the two patients without centrally acting medication (2, 10) evidenced a clear reduction of theta postoperatively.
Discussion
Theta dominance in resting EEG
The most obvious characteristic in the EEG spectra of the patient group was the slowing of the dominant peak and enhanced theta and beta power (Fig. 1), in line with previous publications (Gücer et al., 1978; Llinás et al., 1999; Sarnthein et al., 2003). The average peak frequency of the patient group (8.4 Hz) was below the slowed mean EEG peak frequency reported for normal ageing (Niedermeyer and Lopes da Silva, 1999; McEvoy et al., 2001; Ebersole and Pedley, 2003). Both peak height and peak frequency gave the best discrimination between patient group and control group (Fig. 2). In this way we have substantiated the difference in EEG between neurogenic pain patients and healthy controls on a statistical basis. The general slowing and theta overactivity reported in our patients (Fig. 3) correspond to a constantly altered resting EEG and is viewed as an aspect of the pathophysiology. As a result of our selection criteria, pain locations vary among patients. Our results therefore reflect the pathophysiology of neurogenic pain common to all patients. We have refrained from comparisons in subgroups because of their insufficient size. Finally, our findings might also be related to effects of medication as discussed in the previous section (Table 1). We estimate the effect of medication to be of secondary relevance since theta overproduction occurred also in the unmedicated subgroup of six patients.
TCD
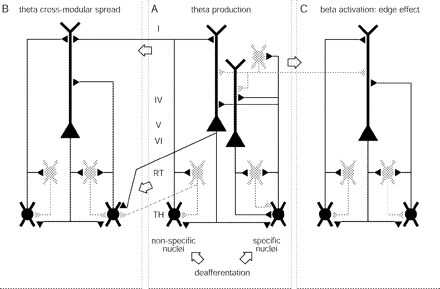
The mechanisms generating resting EEG are still a subject of debate, in particular the role of the thalamus (Nunez et al., 2001). Encouraged by our earlier finding of strong thalamocortical coupling (Sarnthein et al., 2003, p. 65; Sarnthein et al., 2005, p. 114), we propose here an interpretation of the results in the framework of TCD. This thalamocortical concept of chronic neurogenic pain was proposed (Llinás et al., 1999, 2001; Jeanmonod et al., 2001b) on the basis of experimental evidence (Llinás and Jahnsen, 1982; Ribary et al., 1991; Steriade et al., 1997; Steriade, 2001) and the clinical finding of LTS in pain patients (Lenz et al., 1989; Jeanmonod et al., 1993, 1996). It may be characterized using the following sequential set of events (schematized in Fig. 7):
A lesion leads to deafferentation of excitatory inputs on thalamic relay cells and initiates the neurogenic pain syndrome. The lesion may be peripheral or central and may lead to bottom–up deafferentation (Fig. 7A). A cortical lesion may lead to top–down deafferentation. The deafferentation of excitatory inputs results in disfacilitation and cell membrane hyperpolarization.
In the hyperpolarized state, deinactivation of calcium T-channels causes thalamic relay neurons to fire LTS bursts at theta frequency (Llinás and Jahnsen, 1982). Such hyperpolarization is thought to correlate with the state of reduced activity observed by PET in the thalamus of neurogenic pain patients (Iadarola et al., 1995; Hsieh et al., 1995; Nakabeppu et al., 2001; Jones et al., 2003).
Bursting thalamic relay neurons exert a rhythmic influence on thalamocortical loops in the theta frequency band. Thalamic and cortical areas are densely and reciprocally interconnected (Steriade et al., 1997; Jones, 2001). The tight functional coupling between thalamus and cortex is confirmed by the high theta coherence between the two (Sarnthein et al., 2003). This coupling is sustained by thalamocorticothalamic and also by thalamoreticulothalamic and corticoreticulothalamic recurrent projections (Steriade, 2001). The tendency of the thalamocortical network to maintain a given functional modality reinforces the hyperpolarized state over time (Pedroarena and Llinas, 1997).
Divergent thalamocortical, corticothalamic and reticulothalamic projections provide the anatomical substrate for diffusion of low frequency activity to an increasing number of neighbouring thalamocortical loops (Fig. 7B: theta cross-modular spread). This phenomenon may explain the often observed delay between the occurrence of the causal insult and the beginning of pain.
After recruitment of a sufficiently large number of thalamocortical loops, excess theta power becomes measurable in thalamic local field potentials (Sarnthein et al., 2003), MEG (Llinás et al., 1999) and EEG (Fig. 1A). Why do we not observe a sharp EEG spectral peak at the LTS interburst frequency of 4 Hz? LTS exert influence on the thalamocortical system, but the same is true for cortical determinants of the EEG, such as refractory periods and axonal transmission latencies (Nunez et al., 2001). Such determinants are not affected by the neurological disorders of our patients. This may explain why the presence of LTS in patients results in excess EEG oscillations spread over the whole theta band. Furthermore, increased low-frequency oscillations also occur during sleep (Steriade, 2001) and cognitive tasks (Klimesch, 1999; Kahana et al., 2001), where they are considered as normal. It is the continuous and widespread overproduction of slow rhythms in the awake brain that characterizes TCD.
The final step towards the production of neurogenic pain is related to the reciprocal cortico-cortical inhibition mediated by GABAergic interneurons, which is a general feature of cortical organization (Fig. 7C). Thalamo-cortical modules in theta mode exert less collateral inhibition on neighbouring modules, which are thereby overactivated in high (beta) frequencies. This event has been termed edge effect (Llinás et al., 1999). The concept is inspired by the effect of lateral inhibition in the retina. The asymmetrical inhibition between a low frequency cortical area and neighbouring high frequency domains provides a ring of reduced inhibition onto, and thus activation of, the cortex surrounding this low frequency area. Support for such an effect was first provided by the increased interfrequency covariation between theta and beta ranges in MEG (Llinás et al., 2003; Llinás et al., 2005). Recently, the increase of high frequency activation around a core of theta modules could be demonstrated in a slice preparation (Llinás, et al., 2003; Llinás et al., 2005). Also in the thalamus of patients, high interfrequency covariation and bicoherence was found (Sarnthein et al., 2003). We were able to show enhanced bicoherence also in the scalp EEG from patients compared with healthy controls (Fig. 4). The dominance of beta activity in frontal electrodes (Fig. 3) also suggests anterior cortical generators of the scalp EEG, which we currently investigate and which would be consistent with activation of the insulae in the known cerebral network relevant for pain processing (Treede et al., 1999; Jones et al., 2003; Apkarian et al., 2005).
Scheme of thalamocortical circuits relevant for TCD. Shown are three thalamocortical modules which we consider to be identical (Llinás et al., 1999). Module A is depicted in more detail (Jones, 2001). One module includes, first, cortical layers (I, IV, V and VI) with pyramidal cells and one grey GABAergic inhibitory cell, second, nucleus reticularis (RT, grey GABAergic inhibitory cells) and third, thalamus (TH), represented by one specific and one non-specific cell. The specific thalamic cell projects to the apical dendrites of both layer V and VI pyramidal cells and collaterals sustain reticular feedback and cortical feedforward inhibitions. The non-specific thalamic cell projects to RT and only to the layer V pyramidal neuron but has a divergent connection onto the neighbouring module. The corticothalamic feedback connection is depicted as intramodular onto RT and its thalamic relay cell, and divergent intra- and cross-modular onto three thalamic relay cells. There are also divergent cross-modular reticulothalamic projections. The open arrows underline the cross-modular passages through thalamocortical, corticothalamic, reticulothalamic and corticocortical pathways. See the section discussion for details on the dynamics of the TCD.
Effect of CLT
The observed gradual change of the patients' EEG spectra towards the healthy control group spectrum has the following implications. First, it provides further support for our hypothesis that the amount of thalamic LTS burst production finds a correlate in the scalp EEG. Second, the gradual response to the sudden removal of LTS confirms that LTS activity constitutes only one of several determinants of EEG rhythmicity (Nunez et al., 2001). Third, the EEG change over several months allows an estimate of the timeframe for wide range plasticity in the brain and parallels our observation of gradual clinical improvement (Jeanmonod et al., 2001a).
Conclusions
Our data relate the clinical phenomenon of chronic neurogenic pain to slowed rhythmicity of scalp EEG. This relationship is further supported by the reduction of theta power in the EEG of patients after the therapeutic lesion CLT in the medial thalamus. The TCD mechanism offers an explanation of the pathophysiology in linking the function of calcium T-channels in the membrane of thalamic cells to the global properties of scalp EEG power spectra. EEG spectral analysis might in this way serve as an additional tool to diagnose chronic neurogenic pain and to monitor the postoperative development of patients.
The authors thank A. Morel for critical comments on an earlier version of the manuscript, J. Dodd for help with the EEG recordings, V. Bügler for contacting the patients and H. G. Wieser for advice on data analysis. The authors gratefully acknowledge financial support from the Stiftung für wissenschaftliche Forschung an der Universität Zürich, the EMDO foundation and the Mach-Gaensslen foundation.
References
Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health disease.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis.
Dumermuth G, Huber PJ, Kleiner B, Gasser T. Analysis of the interrelations between frequency bands of the EEG by means of the bispectrum. A preliminary study.
Ebersole JS, Pedley TA. Current practice of clinical electroencephalography. Philadephia: Lippincott Williams & Wilkins;
Gücer G, Niedermeyer E, Long DM. Thalamic EEG recordings in patients with chronic pain.
Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography.
Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, et al. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain.
Jeanmonod D, Magnin M, Morel A. Thalamus and neurogenic pain: physiological, anatomical and clinical data.
Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms.
Jeanmonod D, Magnin M, Morel A, Siegemund M. Surgical control of the human thalamocortical dysrhythmia: I. Central lateral thalamotomy in neurogenic pain.
Jeanmonod D, Magnin M, Morel A, Siegemund M, Cancro R, Lanz M, et al. Thalamocortical dysrhythmia II. Clinical and surgical aspects.
Jones AK, Kulkarni B, Derbyshire SW. Pain mechanisms and their disorders.
Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis.
Landolt HP, Retey JV, Tonz K, Gottselig JM, Khatami R, Buckelmuller I, et al. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans.
Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain.
Lindhardt K, Gizurarson S, Stefansson SB, Olafsson DR, Bechgaard E. Electroencephalographic effects and serum concentrations after intranasal and intravenous administration of diazepam to healthy volunteers.
Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro.
Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography.
Llinás R, Ribary U, Jeanmonod D, Cancro R, Kronberg E, Schulman J, et al. Thalamocortical dysrhythmia I. Functional and imaging aspects.
Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices.
Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect.
McEvoy LK, Pellouchoud E, Smith ME, Gevins A. Neurophysiological signals of working memory in normal aging.
Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus.
Nakabeppu Y, Nakajo M, Gushiken T, Tsuchimochi S, Tani A, Kanmura Y. Decreased perfusion of the bilateral thalami in patients with chronic pain detected by Tc-99m-ECD SPECT with statistical parametric mapping.
Niedermeyer E, Lopes da Silva FH. Electroencephalography: basic principles, clinical applications, and related fields. Philadephia: Lippincott Williams & Wilkins;
Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks.
Pedroarena C, Llinas R. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons.
Percival DB, Walden AT. Spectral analysis for physical applications. Cambridge: Cambridge University Press;
Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000).
Pfurtscheller G, Stancak A Jr, Neuper C. Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review.
Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans.
Salinsky MC, Oken BS, Morehead L. Test-retest reliability in EEG frequency analysis.
Salinsky MC, Oken BS, Storzbach D, Dodrill CB. Assessment of CNS effects of antiepileptic drugs by using quantitative EEG measures.
Sarnthein J, Morel A, von Stein A, Jeanmonod D. Thalamic theta field potentials and EEG: high thalamocortical coherence in patients with neurogenic pain, epilepsy and movement disorders.
Sarnthein J, Morel A, von Stein A, Jeanmonod D. Thalamocortical theta coherence in neurological patients at rest and during a working memory task.
Schack B, Vath N, Petsche H, Geissler HG, Möller E. Phase-coupling of theta-gamma EEG rhythms during short-term memory processing.
Steriade M. Impact of network activities on neuronal properties in corticothalamic systems.
Steriade M, Jones EG, McCormick DA. Thalamus: organisation and Function. Vol. 1. Oxford: Elsevier;
Author notes
1Universitätsspital, Funktionelle Neurochirugie, CH-8091 Zürich, 2Center for Integrative Human Physiology and 3Biostatistik, Universität Zürich, Switzerland