Abstract
Background
It has been determined that the chemokine receptor CXCR4 and its ligand stromal cell-derived factor-1 (SDF-1) regulate several key processes in a wide variety of cancers. However, the function and mechanism of the SDF-1/CXCR4 system in the metastasis of colorectal cancer remain controversial.
Methods
Immunohistochemistry was performed to examine quantitatively the expression of CXCR4 in 40 human samples of colorectal cancer and liver metastasis. The functions of SDF-1 on HCT116 colon cancer cells were investigated in vitro. We subcutaneously inoculated HCT116 cells with hepatic stellate cells (HSCs) expressing SDF-1. The CXCR4 inhibitor AMD3100 was tested in vitro and in vivo.
Results
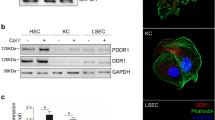
By quantitatively counting the number of cells, it was shown that there are more CXCR4-positive cells at the metastatic site in the liver compared with the primary sites. We demonstrated the effect of SDF-1 on the invasion and antiapoptosis of HCT116 cells in vitro. In mouse experiment of liver metastasis, intraperitoneal administration of AMD3100 blocked the metastatic potential of HCT116 cells. Furthermore, we found that α-smooth muscle actin (α-SMA)-positive myofibroblasts derived from HSCs, surrounding the liver metastasis foci, secreted SDF-1. The subcutaneous inoculation of HCT116 cells with HSCs promoted the tumor initiation in nude mice, indicating the importance of the direct interaction between these cells in vivo.
Conclusion
These results suggest that HSCs play important role in liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis and provide preclinical evidence that blockade of the axis is a target for antimetastasis therapy.





Similar content being viewed by others
References
Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–27.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96.
Taichman RS, Cooper C, Keller ET, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–7.
Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6.
Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345(11):833–5.
Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63(13):3833–9.
Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6(9):3530–5.
Kijima T, Maulik G, Ma PC, et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62(21):6304–11.
Geminder H, Sagi-Assif O, Goldberg L, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167(8):4747–57.
Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23(12):2744–53.
Ottaiano A, Franco R, Aiello Talamanca A, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12(9):2795–803.
Schimanski CC, Schwald S, Simiantonaki N, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11(5):1743–50.
Iimuro Y, Nishio T, Morimoto T, et al. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124(2):445–58.
Thompson KC, Trowern A, Fowell A, et al. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation in vitro. Hepatology. 1998;28(6):1518–24.
Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50(19):6130–8.
Bouvet M, Tsuji K, Yang M, et al. In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res. 2006;66(23):11293–7.
Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–9.
Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250(2):273–83.
Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48.
Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 1997;430(3):195–207.
Ara T, Tokoyoda K, Okamoto R, et al. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105(8):3155–61.
Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14(3):171–9.
Ottaiano A, di Palma A, Napolitano M, et al. Inhibitory effects of anti-CXCR4 antibodies on human colon cancer cells. Cancer Immunol Immunother. 2005;54(8):781–91.
Raman D, Baugher PJ, Thu YM, et al. Role of chemokines in tumor growth. Cancer Lett. 2007;256(2):137–65.
Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64(23):8604–12.
Kawada K, Hosogi H, Sonoshita M, et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26(32):4679–88.
Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23.
Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–7.
Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32.
Shimizu S, Yamada N, Sawada T, et al. In vivo and in vitro interactions between human colon carcinoma cells and hepatic stellate cells. Jpn J Cancer Res. 2000;91(12):1285–95.
Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9):1391–402.
Struckmann K, Mertz K, Steu S, et al. pVHL co-ordinately regulates CXCR4/CXCL12 and MMP2/MMP9 expression in human clear-cell renal cell carcinoma. J Pathol. 2008;214(4):464–71.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsusue, R., Kubo, H., Hisamori, S. et al. Hepatic Stellate Cells Promote Liver Metastasis of Colon Cancer Cells by the Action of SDF-1/CXCR4 Axis. Ann Surg Oncol 16, 2645–2653 (2009). https://doi.org/10.1245/s10434-009-0599-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0599-x