Summary
The isoenzymes which catalyse the polymorphic hydroxylations of debrisoquine/sparteine and S-mephenytoin are cytochromes P450 2D6 and P450 2C19 (CYP2D6 and CYP2C19), respectively. CYP2D6 is involved in the stereospecific metabolism of several important groups of drugs, for example antiarrhythmics, antidepressants and neuroleptics.
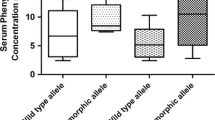
About 7% of Caucasians but only 1 % of Orientals are poor metabolisers (PMs) of debrisoquine. The most common mutated allele CYP2D6B in Caucasian PMs is almost absent from their Oriental counterparts. On the other hand, the mean activity of CYP2D6 in Oriental extensive metabolisers (EMs) is lower than that in Caucasian EMs. This is due to the frequent distribution of a partially deficient CYP2D6 allele causing a Pro34 → Ser amino acid exchange in as many as 50% of Oriental alleles. This is the molecular genetic basis for the slower metabolism of antidepressants and neuroleptics observed in Oriental compared with Caucasian people, and consequently for the lower dosages of these drugs used.
While CYP2D6 catalyses the metabolism of lipophilic bases only, CYP2C19 is involved in the metabolism of acids (e.g. S-mephenytoin), bases (e.g. imipramine and omeprazole) and neutral drugs (e.g. diazepam). About 3% of Caucasians and 12 to 22% of Orientals are PMs of S-mephenytoin. Polymerase chain reaction-based genotyping techniques recently became available for the two CYP2C19 mutated alleles m1 and m2, which cause no enzyme to be expressed, m1 accounts for about 80% of the mutations responsible for the PM phenotypes in Caucasian, Oriental and Black people.
Diazepam is partially demethylated by CYP2C19, and the high frequency of mutated alleles in Orientals is probably the reason why such populations have a slower metabolism and are treated with lower doses of diazepam than Caucasians. Omeprazole is to a major extent hydroxylated by CYP2C19, and there is an approximately 10-fold difference in oral clearance between EMs and PMs of S-mephenytoin.
The separation of Caucasians from Orientals is fairly recent in the evolutionary process (40 000 to 60 000 years ago); the separation of Black from Caucasian/ Oriental people occurred much earlier, about 150 000 years ago. As pronounced differences have been found between Caucasians and Orientals in the CYP2D6 and CYP2C19 enzymes, it might be expected that Black people will show even greater differences in this respect. Some studies have been performed with Black participants, but the picture is not clear. The mean CYP2D6 activity in Black EMs seems to be lower than that in Caucasian EMs and similar to that of Oriental EMs. The incidence of poor metabolism of S-mephenytoin in Black people is 4 to 7%, i.e. between that of Caucasians and Orientals. The major mutation m1 which causes the PM phenotype is the same in these 3 races, and is thus a very ancient one.
Similar content being viewed by others
References
Kalow W. Ethnic differences in drug metabolism. Clin Pharmacokinet 1982; 7: 373–400
Kalow W, Bertilsson L. Interethnic factors affecting drug response. In: Testa B, Meyer UA, editors. Advances in drug research. Vol. 25, London: Academic Press 1994: 1–53
Wood AJJ, Zhou HH. Ethnic differences in drug disposition and responsiveness. Clin Pharmacokinet 1991; 20: 350–73
Lou YC. Differences in drug metabolism polymorphisms between Orientals and Caucasians. Drug Metab Rev 1990; 22: 451–75
Lin K-M, Poland RE, Nakasaki G, editors. Psychopharmacology and psychobiology of ethnicity. Washington DC: American Psychiatric Press, 1993
Bertilsson L, Dahl M-L, Ingelman-Sundberg M, et al. Interindividual and interethnic differences in polymorphic drug oxidation — implications for drug therapy with focus on psychoactive drugs. In: Pacifici GM, Fracchia GN, editors. Advances in drug metabolism in man. Brussels: European Commission, 1995: 85–136
Kalow W, editor. Pharmacogenetics of drug metabolism. New York: Pergamon Press, 1992
Evans DAP. Genetic factors in drug therapy. Clinical and molecular pharmacogenetics. Cambridge: Cambridge University Press, 1993
Vogel F, Motulsky AG. Human genetics. Problem and approaches, 2nd ed. Berlin: Springer-Verlag, 1986
Nei M, Saitou N. Genetic relationship of human populations and ethnic differences in reactions to drugs and food. In: Kalow W, Goedde HW, Agarwal DP, editors. Ethnic differences in reactions to drugs and xenobiotics. New York: Alan R. Liss, 1986: 21–37
Kalow W. Pharmacoanthropology and the genetics of drug metabolism. In: Kalow W, editor. Pharmacogenetics of drug metabolism. New York: Pergamon Press, 1992; 865–77
Gonzalez FJ. Human cytochromes P450: problems and prospects. Trends Pharmacol Sci 1992; 13: 346–52
Ingelman-Sundberg M, Johansson I. The molecular genetics of the human drug metabolizing cytochrome P450s. In: Pacifici GM, Fracchia GN, editors. Advances in drug metabolism in man. Brussels: European Commission, 1995: 543–85
Mahgoub A, Idle JR, Dring DG, et al. Polymorphic hydroxyla-tion of debrisoquine in man. Lancet 1987; 2: 584–6
Eichelbaum M, Spannbrucker N, Steinke B, et al. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharmacol 1979; 16: 183–7
Eichelbaum M, Bertilsson L, Sawe J, et al. Polymorphic oxidation of sparteine and debrisoquine. Related pharmacogenetic entities. Clin Pharmacol Ther 1982; 31: 184–6
Horai Y, Taga J, Ishizaki T, Ishikawa K. Correlations among the metabolic ratios of three test probes (metoprolol, debrisoquine and sparteine) for genetically determined oxidation polymorphism in a Janpaneses population. Br J Clin Pharmacol 1990; 29: 111–5
Woolhouse NM, Eichelbaum M, Oates NS, et al. Dissociation of coregulatory control of debrisoquine/phenformin and sparteine oxidation in Ghanians. Clin Pharmacol Ther 1985; 37: 512–21
Evans DA, Mahgoub A, Sloan TP, et al. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet 1980; 17: 102–5
Alvan G, Bechtel P, Iselius L, et al. Hydroxylation polymorphisms of debrisoquine and mephenytoin in European populations. Eur J Clin Pharmacol 1990; 39: 533–7
Bertilsson L, Lou YQ, Du YL, et al. Pronounced differences between native Chinese and Swedish populations in the polymorphic hydroxylations of debrisoquine and S-mephenytoin. Clin Pharmacol Ther 1992; 51: 388–97
Eichelbaum M, Gross AS. The genetic polymorphism of debriosquine/sparteine metabolism — clinical aspects. In: Kalow W, editor. Pharmacogenetics of drug metabolism. New York: Pergamon Press, 1992: 625–48
Nakamura K, Goto F, Ray WA, et al. Interethnic differences in genetic polymorphism of debrisoquin and mephenytoin hydroxylation between Japanese and Caucasian populations. Clin Pharmacol 1985; 38: 402–8
LLerena A, Edman G, Cobaleda J, et al. Relationship between personality and debrisoquine hydroxylation capacity — suggestion of an endogenous neuroactive substrate or product of the cytochrome P4502D6. Acta Psychiatr Scand 1993; 87: 23–8
Lou Y-C, Liu Y, Bertilsson L, et al. Low frequency of slow debrisoquine hydroxylation in a native Chinese population. Lancet 1987; ii: 852–3
Mbanefo C, Bababunmi EA, Mahgoub A, et al. A study of the debrisoquine hydroxylation polymorphism in a Nigerian population. Xenobiotica 1980; 10: 811–8
Iyun AO, Lennard MS, Tucker GT, et al. Metoprolol and debrisoquine metabolism in Nigerians: lack of evidence for polymorphic oxidation. Clin Pharmacol Ther 1986; 40: 387–94
Kalow W. Interethnic variation of drug metabolism. Trends Pharmacol Sci 1991; 12: 102–7
Sommers DK, Moncrieff J, Avenant J. Non-correlation between debrisoquine and metoprolol polymorphisms in the Venda. Hum Toxicol 1989; 8: 365–8
Eichelbaum M, Baur MP, Dengler HJ, et al. Chromosomal assignment of human cytochrome P450 (debrisoquine/sparteine type) to chromosome 22. Br J Clin Pharmacol 1987; 23: 455–8
Skoda RC, Gonzales FJ, Demierre A, et al. Two mutant alleles of the human cytochrome P450 dbl gene (P450 II Dl) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci USA 1988; 85: 5240–3
Gaedigk A, Blum M, Gaedigk R, et al. Deletion of the entire cytochrome P450 CYP2D6 gene as a cause of impaired drug metabolism in poor metabolizers of the debrisoquine/sparteine polymorphism. Am J Hum Genet 1991; 48: 943–50
Heim MH, Meyer UA. Evolution of a highly polymorphic human cytochrome P450 gene cluster: CYP2D6. Genomics 1992; 14: 49–58
Heim M, Meyer UA. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet 1990; 336: 529–32
Broly F, Gaedigk A, Heim M, et al. Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol 1991; 10: 545–58
Dahl M-L, Johansson I, Porsmyr Palmertz M, et al. Analysis of the CYP2D6 gene in relation to debrisoquine and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther 1992; 51: 12–7
Johansson I, Yue QY, Dahl M-L, et al. Genetic analysis of the interethnic difference between Chinese and Caucasians in the polymorphic metabolism of debrisoquine and codeine. Eur J Clin Pharmacol 1991; 40: 553–6
Johansson I, Oscarsson M, Yue QY, et al. Genetic analysis of the Chinese CYP2D6 locus. Characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol Pharmacol 1994; 46: 452–9
Wang SL, Huang JD, Lai MD, et al. Molecular basis of genetic variation in debrisoquine hydroxylation in Chinese subjects: polymorphism in RFLP and DNA sequence of CYP2D6. ClinPharmacol Ther 1993; 53: 410–8
Evans WE, Relling MV, Rahman A, et al. Genetic basis for a lower prevalence of deficient CYP2D6 oxidative drug metabolism phenotypes in black Americans. J Clin Invest 1993; 91: 2150–4
Masimirembwa CM, Johansson I, Hasler JA, et al. Genetic polymorphism of cytochrome P450 2D6 in Zimbabwean population. Pharmacogenetics 1993; 3: 275–80
Yue QY, Svensson JO, Alm C, et al. Interindividual and interethnic differences in the demethylation and glucuronidation of codeine. Br J Clin Pharmacol 1989; 28: 629–37
Yue QY, Bertilsson L, Dahl-Puustinen M-L, et al. Disassociation between debrisoquine hydroxylation phenotype and genotype among Chinese. Lancet 1989; ii: 870
Yokota H, Tamura S, Furuya H, et al. Evidence for a new variant CYP2D6 allele CYP2D6J in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics 1993; 3: 256–63
Bertilsson L, Åberg-Wistedt A, Gustafsson LL, et al. Extremely rapid hydroxylation of debrisoquine — a case report with implication for treatment with nortriptyline and other tricyclic antideprassants. Ther Drug Monit 1985; 7: 478–80
Bertilsson L, Dahl M-L, Sjöqvist F, et al. Molecular basis for rational megaprescribing in ultrarapid hydroxylators of debrisoquine. Lancet 1993; 341: 63
Johansson I, Lundqvist E, Bertilsson L, et al. Inherited amplification of an active gene in the cytochrome P450 CYP2D-locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci USA 1993; 90: 11825–9
Dahl M-L, Johansson I, Bertilsson L, et al. Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. J Pharmacol Exp Ther 1995; 274: 516–20
Smith DA, Jones BC. Speculations on the substrate structure-activity relationship (SSAR) of cytochrome P450 enzymes. Biochem Pharmacol 1992; 44: 2089–98
Koymans L, Vermeiden NP, Van Acker SA, et al. A predictive model for substrates of cytochrome P450-debrisoquine (2D6). Chem Res Toxicol 1992; 5: 211–9
Bertilsson L, Åberg-Wistedt A. The debrisoquine hydroxylation test predicts steady-state plasma levels of desipramine. Br J Clin Pharmacol 1983; 15: 388–90
Steiner E, Iselius L, Alvan G, et al. A family study of genetic and environmental factors determining polymorphic hydroxylation of debrisoquine. Clin Pharmacol Ther 1985; 38: 394–401
Spina E, Steiner E, Ericsson Ö, et al. Hydroxylation of des-methyldesipramine dependence on the debrisoquin hydroxylation phenotype. Clin Pharmacol Ther 1987; 41: 314–9
Rudorfer MV, Lane EA, Chang WH, et al. Desipramine pharmacokinetics in Chinese and Caucasian volunteers. Br J Clin Pharmacol 1984; 17: 433–40
Lin KM, Poland RE, Smith MW, et al. Pharmacokinetic and other related factors affecting psychotropic responses in Asians. Psychopharmacol Bull 1991; 27: 427–39
LLerena A, Dahl M-L, Ekqvist B, et al. Haloperidol disposition is dependent on the debrisoquine hydroxylation phenotype: increased plasma levels of the reduced metabolite in poor metabolizers. Ther Drug Monit 1992; 14: 261–4
Nyberg S, Farde L, Halldin C, et al. D2 dopamine receptor occupancy during low-dose treatment with haloperidol decanoate. Am J Psychiatry 1995; 152: 173–8
Dahl M-L, Bertilsson L. Genetically variable metabolism of antidepressants and neuroleptic drugs in man. Pharmacogenetics 1993; 3: 61–70
Sjöqvist F. Pharmacogenetic factors in the metabolism of tricyclic antidepressants and some neuroleptics. In: Kalow W, editor. Pharmacogenetics of drug metabolism. New York: Pergamon Press, 1992: 689–700
Dahl M-L, LLerena A, Bondesson U, et al. Disposition of clozapine in man: lack of association with debrisoquine and S-mephenytoin hydroxylation polymorphisms. Br J Clin Pharmacol 1994; 37: 71–4
Bertilsson L, Carrillo JA, Dahl M-L, et al. Clozapine disposition covaries with CYP1A2 activity determined by a caffeine test. Br J Clin Pharmacol 1994; 38: 471–3
Lin KM, Finder E. Neuroleptic dosage for Asians. Am J Psychiatry 1983; 140: 490–1
Potkin SG, Shen Y, Pardes H, et al. Haloperidol concentrations elevated in Chinese patients. Psychiatry Res 1984; 12: 167–72
Lin KM, Poland RE, Lau JK, et al. Haloperidol and prolactin concentrations in Asians and Caucasians. J Clin Phycho-pharmacol 1988; 8: 195–201
Bertilsson L, Alm C, de las Carreras C, et al. Debrisoquine hydroxylation polymorphism and personality. Lancet 1989; 1: 555
Kalow W, Tyndale RF. Debrisoquine/sparteine monooxygenase and other P450s in brain. In: Kalow W, editor. Pharmacogenetics of drug metabolism. New York: Pergamon Press, 1992: 649–56
Niznik HB, Tyndale RF, Sallee RF, et al. The dopamine transporter and cytochrome P450IID1 (debrisoquine 4-hydroxyl-ase) in brain: resolution and identification of two distinct [3H] GBR-12935 binding proteins. Arch Biochem Biophys 1990; 276: 424–32
Brosen K, Sindrup SH, Skjelbo, et al. Role of genetic polymorphism in psychopharmacology — an update. In: Gram LF, Balant LP, Meltzer HY, et al., editors. Clinical pharmacology in psychiatry. Berlin and Heidelberg: Springer-Verlag 1993: 199–211
Küpfer A, Bircher J, Preisig R. Stereoselective metabolism, pharmacokinetics and biliary elimination of phenylethyl-hydantoin (Nirvanol) in the dog. J Pharmacol Exp Ther 1977; 203: 493–9
Küpfer A, Desmond P, Schenker S, et al. Family study of a genetically determined deficiency of mephenytion hydroxylation in man. Pharmacologist 1979; 21: 173
Wilkinson GR, Guengerich FP, Branch RA. Genetic polymorphism of S-mephenytoin hydroxylation. In: Kalow W, editor. Pharmacogenetics of drug metabolism. New York: Pergamon Press, 1992: 657–85
Wedlund PJ, Aslanian WS, McAllister CB, et al. Mephenytoin hydroxylation deficiency in Caucasians: frequency of a new oxidative drug metabolism polymorphism. Clin Pharmacol Ther 1984; 36: 773–80
Sanz EJ, Villen T, Alm C, et al. S-mephenytoin hydroxylation phenotype in a Swedish population — determined after co-administration with debrisoquin. Clin Pharmacol Ther 1989; 45: 495–9
Tybring G, Bertilsson L. A methodological investigation on the estimation of the S-mephenytoin hydroxylation phenotype using the urinary S/R ratio. Pharmacogenetics 1992; 2: 241–3
Wedlund PJ, Sweetman BJ, Wilkinson GR, et al. Pharmacogenetic association between the formation of 4-hydroxy-mephenytoin and a new metabolite of S-mephenytoin in man. Drug Metabol Disp 1987; 15: 277–9
Jurima M, Inaba T, Kadar D, et al. Genetic polymorphism of mephenytoin p(4′)hydroxylation: difference between Orientals and Caucasians. Br J Clin Pharmacol 1985; 19: 483–7
Horai Y, Nakano M, Ishizaki T, et al. Metoprolol and mephenytoin oxidation polymorphisms in Far Eastern Oriental subjects: Japanese versus mainland Chinese. Clin Pharmacol Ther 1989; 46: 198–207
Sohn DR, Kusaka M, Ishizaki T, et al. Incidence of S-mephenytoin hydroxylation deficiency in a Korean population and the interphenotypic differences in diazepam pharmacokinetics. Clin Pharmacol Ther 1992; 52: 160–9
Pollock BG, Perel JM, Kirshner M, et al. S-mephenytoin 4-hy-droxylation in older Americans. Eur J Clin Pharmacol 1991; 40: 609–11
Masimirembwa CM, Bertilsson L, Johansson I, et al. Phenotyping and genotyping of S-mephenytoin hydroxylase (Cytochrome P450 2C19) in a Shona population of Zimbabwe. Clin Pharmacol Ther 1995; 57: 656–61
Goldstein JA, de Morais SMF. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 1994; 4: 285–99
Wrighton SA, Stevens JC, Becker GW, et al. Isolation and characterization of human liver cytochrome P4502C19: correlation between 2C19 and S-mephenytoin 4′-hydroxylation. Arch Biochem Biophys 1993; 306: 240–5
Goldstein JA, Faletto MB, Romkes-Sparks M, et al. Evidence that CYP2C19 is the major (S)-mephenytoin 4′-hydroxylase in humans. Biochemistry 1994; 33: 1743–52
De Morais SMF, Wilkinson GR, Blaisdell J, et al. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 1994; 269: 15419–22
De Morais SMF, Wilkinson GR, Blaisdell J, et al. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 1994; 46: 594–8
Skjelbo E, Brosen K, Hallas J, et al. The mephenytoin oxidation polymorphism is partially responsible for the N-demethylation of imipramine. Clin Pharmacol Ther 1991; 49: 18–23
Ward SA, Walle T, Walle UK, et al. Propranolol’s metabolism is determined by both mephenytoin and debrisoquine hydroxylase activities. Clin Pharmacol 1989; 45: 72–9
Ward SA, Helsby NA, Skjelbo E, et al. The activation of the biguanide antimalarial proguanil co-segregates with the mephenytoin oxidation polymorphism — a panel study. Br J Clin Pharmacol 1991; 31: 689–92
Inaba T, Jurima M, Mahon WA, et al. In vitro inhibition studies of two isozymes of human liver cytochrome P-450. Mephenytoin p-hydroxylase and sparteine monooxygenase. Drug Metab Dispos 1985; 13: 443–8
Bertilsson L, Henthorn TK, Sanz E, et al. Importance of genetic factors in the regulation of diazepam metabolism: Relationship to S-mephenytoin but not debrisoquin hydroxylation phenotype. Clin Pharmacol Ther 1989; 45: 348–55
Zhang Y, Reviriego J, Lou YQ, et al. Diazepam metabolism in native Chinese poor and extensive hydroxylators of S-mephenytoin: interethnic differences in comparison with white subjects. Clin Pharmacol Ther 1990; 48: 496–502
Sohn DR, Kusaka M, Ishizaki T, et al. Incidence of S-mephenytoin hydroxylation deficiency in a Korean population and the interphenotypic differences in diazepam pharmacokinetics. Clin Pharmacol Ther 1992; 52: 160–9
Bertilsson L, Kalow W. Why are diazepam metabolism and polymorphic S-mephenytoin hydroxylation associated with each other in white and Korean populations, but not in Chinese populations? Clin Pharmacol Ther 1993; 53: 608–10
Ghoneim MM, Korttila K, Chiang CK, et al. Diazepam effect and kinetics in Caucasians and Orientals. Clin Pharmacol Ther 1981; 29: 749–56
Kumana CR, Lauder IJ, Chan M, et al. Differences in diazepam pharmacokinetics in Chinese and white Caucasians — relation to body lipid stores. Eur J Clin Pharmacol 1987; 32: 211–5
Perucca E, Gatti G, Cipolla G, et al. Inhibition of diazepam metabolism by fluvoxamine: a pharmacokinetic study in normal volunteers. Clin Pharmacol Ther 1994; 56: 471–6
Brøsen K, Skjelbo E, Rasmussen, et al. Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol 1993; 45: 1211–4
Anderssson T, Cederberg C, Edvardsson G, et al. Effect of omeprazole treatment on diazepam plasma levels in slow versus normal rapid metabolisers of omeprazole. Clin Pharmacol Ther 1990; 47: 79–85
Andersson T, Regardh CG, Dahl-Puustinen M-L, et al. Slow omeprazole metabolisers are also poor S-mephenytoin hydroxylators. Ther Drug Monit 1990; 12: 415–6
Andersson T, Regardh C-G, Lou Y-C, et al. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics 1992; 2: 25–31
Sohn DR, Kobayashi K, Chiba K, et al. Disposition kinetics and metabolism of omeprazole in extensive and poor metabolisers of S-mephenytoin 4′-hydroxylation recruited from an Oriental population. J Pharmacol Exp Ther 1992; 262: 1195–202
Ishizaki T, Sohn DR, Kobayashi K, et al. Interethnic differences in omeprazole metabolism in the two S-mephenytoin hydroxylation phenotypes studied in Caucasians and Orientals. Ther Drug Monit 1994; 16: 214–5
Chang M, Tybring G, Dahl M-L, et al. Interphenotype differences in disposition and effect on gastrin levels of omeprazole-suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol 1995; 39: 511–8
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bertilsson, L. Geographical/Interracial Differences in Polymorphic Drug Oxidation. Clin-Pharmacokinet 29, 192–209 (1995). https://doi.org/10.2165/00003088-199529030-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199529030-00005