Published online Apr 7, 2011. doi: 10.3748/wjg.v17.i13.1725
Revised: August 6, 2010
Accepted: August 13, 2010
Published online: April 7, 2011
AIM: To determine if natural killer T cell (NKT) populations are affected in nonalcoholic fatty liver disease (NAFLD).
METHODS: Patients undergoing bariatric surgery underwent liver biopsy and blood sampling during surgery. The biopsy was assessed for steatosis and immunocyte infiltration. Intrahepatic lymphocytes (IHLs) were isolated from the remainder of the liver biopsy, and peripheral blood mononuclear cells (PBMCs) were isolated from the blood. Expression of surface proteins on both IHLs and PBMCs were quantified using flow cytometry.
RESULTS: Twenty-seven subjects participated in this study. Subjects with moderate or severe steatosis had a higher percentage of intrahepatic CD3+/CD56+ NKT cells (38.6%) than did patients with mild steatosis (24.1%, P = 0.05) or those without steatosis (21.5%, P = 0.03). Patients with moderate to severe steatosis also had a higher percentage of NKT cells in the blood (12.3%) as compared to patients with mild steatosis (2.5% P = 0.02) and those without steatosis (5.1%, P = 0.05).
CONCLUSION: NKT cells are significantly increased in the liver and blood of patients with moderate to severe steatosis and support the role of NKT cells in NAFLD.
- Citation: Adler M, Taylor S, Okebugwu K, Yee H, Fielding C, Fielding G, Poles M. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J Gastroenterol 2011; 17(13): 1725-1731
- URL: https://www.wjgnet.com/1007-9327/full/v17/i13/1725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i13.1725
With the epidemic of obesity burgeoning across much of the world, nonalcoholic fatty liver disease (NAFLD) has become an increasingly pressing problem. The prevalence of NAFLD in western countries is as high as 17%-33%[1], and the more severe form of NAFLD, non-alcoholic steatohepatitis (NASH), will progress to cirrhosis in 20% of patients[1]. Due to its increasing prevalence, NAFLD has become the third leading indication for liver transplantation[2].
The etiopathogenesis of NAFLD/NASH involves a number of environmental, genetic, and inflammatory influences. However, many of the factors that play a role in the development of NAFLD and NASH remain unknown. What is clear is that NASH is associated with hepatic infiltration of inflammatory cells, resulting in hepatocyte injury and hepatocyte death. The prevailing two-hit theory, posited by Day et al[3], proposes an initial hit whereby obesity is associated with hepatic accumulation of free fatty acids and triglycerides, followed by a second hit, whereby oxidative stress, mitochondrial dysfunction, elaboration of pro-inflammatory cytokines and inflammatory cell infiltration leads to development of NASH.
Natural killer T (NKT) cells are a highly conserved subset of lymphocy tes with properties of both T cells (CD3+ expression) and NK cells (CD56+ and CD161+ expression)[4] and have been implicated in NAFLD. NKT cells are concentrated in the liver where they serve an important role in innate immunity. In the murine liver, NKT cells comprise 30%-50% of all hepatic lymphocytes[5]. These cells can be directly cytotoxic via FasL-dependent and perforin-mediated mechanisms, but also produce an array of cytokines that direct cytokine secretion by other cells within their microenvironment[5]. These functions may be responsible for cell death seen in NAFLD. NKT cells are believed to be primarily stimulated by various glycolipids, which are presented by CD1d, an MHC-like molecule on antigen presenting cells, such as Kupffer cells, to the NKT cells’ invariant T cell receptor[6]. The role of NKT cells in immunity has yet to be fully elucidated and there have been many proposed functions for this unique cell, ranging from antitumor activity to autoimmune diseases[7]. In addition, murine models of obesity and fatty liver disease, using leptin-deficient, ob/ob mice, have suggested that NAFLD is associated with depletion of NKT cells[8]. The loss of CD4-expressing NKT cells is particularly intriguing as this cell subset is believed to primarily secrete Th2-type cytokines, including IL-4 and IL-13[9]. This loss of Th2 cytokines might tip the inflammatory milieu of the liver into a pro-inflammatory Th1 state, leading to excessive production of TNF-α and IFN-γ. The increase in pro-inflammatory cytokines likely plays a role in hepatic oxidative stress and recruitment of additional inflammatory cells into the liver, resulting in NASH[10]. The transfer of NKT lymphocytes back into leptin deficient mice has been shown to reduce hepatic steatosis and improves glucose intolerance[11]. In addition, inducing expansion of the NKT cell population, by norepinephrine injection or by stimulation with glucocerebroside, has also been shown to reduce hepatotoxicity and improve hepatic fat content in murine models[12,13].
While murine models of NAFLD clearly support a pivotal role of NKT cells in pathogenesis, data on the role of NKT cells in human NAFLD is limited. Xu and colleagues found that peripheral blood NKT cells are depleted in patients with clinically diagnosed NALFD[14]. Three other studies evaluated intrahepatic NKT cells and had differing results. The study by Kremer et al[15] found that NKT cells are depleted with increased steatosis, whereas the one by Tajiri and colleagues found an increase in NKT cells with steatosis[16]. Finally a study by Syn et al[17] also found an increase in NKT cells with steatosis. In this study, we sought to further investigate the changes in lymphocyte populations that occur in NAFLD.
From January to November 2007, peripheral blood and hepatic tissue were collected from obese subjects undergoing laparoscopic gastric banding surgery. Patients were excluded if they were under the age of 18, infected with hepatitis B virus, hepatitis C virus, HIV, were known to have pre-existing hepatic disease, or found to have any non-NAFLD pathological processes found on histological examination of the liver biopsy material. Patients were also excluded if they had a known history of excessive alcohol ingestion. All enrolled subjects signed an informed consent form that was approved by the institutional review board of NYU Langone Medical Center.
Immediately prior to surgery, 10 mL of blood was obtained from each subject by venipuncture. During the surgery a 2 cm3 liver scissor biopsy was obtained. The liver biopsy sample was placed in 15cc of sterile RPMI 1640 (Mediatech Inc, Herndon, VA) and was transported to the laboratory with the blood sample for lymphocyte isolation. An additional portion of the biopsy was evaluated by a single hepatopathologist who made the diagnosis of NAFLD and NASH using the staging system proposed by Brunt et al[18]. Mild steatosis was defined as steatosis involving up to 33% of hepatocytes, moderate steatosis involved 33%-66% of hepatocytes, and severe steatosis involved greater than 66% of hepatocytes. Steatohepatitis was defined by a number of features including steatosis, ballooning, and acinar and portal inflammation.
Once transported to the laboratory, the liver biopsy sample was washed in sterile phosphate-buffered saline (PBS) and was minced to 1 mm3 pieces in a petri dish with 30 mL of RPMI 1640 containing 0.5 mg/mL collagenase type II (Clostridiopeptidase A), 0.02 mg/mL DNase I, 100 U/mL penicillin, 100 mg/mL streptomycin and 2 mmol/l L- glutamine (all from Sigma-Aldrich, St. Louis, MO) and 10% fetal calf serum (FCS) (Invitrogen, Carlsbad CA). The minced liver was incubated in this digestion solution at 37°C for 30 min after which it was strained through a 70 mm disposable plastic strainer. Immediately after isolation, cells were washed and re-suspended in PBS. The cell solution was then pipetted onto a 30%-70% percoll (Sigma-Aldrich, St. Louis MO) gradient and was centrifuged at 2000 r/min for 30 min. The isolated lymphocytes were removed from the gradient, washed with PBS, resuspended in 1 mL PBS/10% FCS containing 2% formaldehyde (Sigma-Aldrich, St. Louis MO) and placed at 4°C. Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation on a Ficoll-Hypaque density gradient (Mediatech, Herndon, VA).
Cell surface expression of lymphocyte antigens was identified by monoclonal antibody staining of freshly isolated IHLs and PBMCs, followed by flow cytometry using a BD LSR II (Becton Dickinson Immunocytometry Systems (BDIS), Mountain View CA) flow cytometer with analysis using CellQuest® software (BDIS, Mountain View CA). Monoclonal antibodies used in this study included anti-human CD3 (clone UCHT1) (BDIS, Mountain View, CA), anti-human CD4 (clone RPA T4) (Pharmingen, San Diego, CA), anti-human CD8 (clone RPA T8) (Pharmingen, San Diego, CA), anti-human CD56 (clone NCAM16.2) (Pharmingen, San Diego, CA), anti-human CD161 (clone DX12) (Pharmingen, San Diego, CA), anti-human vα24 (clone C15) (Immunotech, Fullerton, CA), and the appropriate isotype controls. During flow cytometry, lymphocytes, initially identified by their forward and side scatter characteristics, were subject to phenotypic analysis. Dead cells were excluded from analysis using 7-aminoactinomycin D (Calbiochem, La Jolla, CA).
IFN-γ and IL-4 secretion by intrahepatic and peripheral blood lymphocytes was determined by ELISA (BD Pharmingen) after culture for 12 h. For these assays, 1 × 105 lymphocytes derived from the liver or blood were co-cultured with monocyte-derived macrophages in the presence of alpha-galactosyl ceramide at 10 μg/mL in a 96-well flat-bottom plate.
Values are expressed as mean ± SD. Statistical comparisons were made between PBMCs and IHLs from individuals using a paired t-test. Statistical comparisons were made between subjects without hepatic steatosis, those with mild steatosis and those with moderate-to-severe steatosis using a two-sample, unequal variance t-test. All reported P values were two-sided at the 0.05 significance level using SPSSTM 11.0 for Windows software (SPSS, Chicago, IL).
Table 1 describes the clinical characteristics of the 27 patients enrolled in this study. Ten of the twenty-seven subjects (37%) had normal liver biopsies, without steatosis, while 11 of 27 (41%) had mild hepatic steatosis and 6 of 27 (22%) had moderate-severe hepatic steatosis. Of the patients with mild steatosis, 10 had increased hepatic lymphocyte infiltration, but were not felt to be severe enough to merit a diagnosis of NASH. One of the six subjects with moderate-severe steatosis had grade 3 steatohepatitis with grade 1 fibrosis. Seventy-eight percent of the subjects were female, their mean age was 42 years, and their mean body mass index (BMI) was 42.9. There were no significant differences between patient cohorts with regard to age, gender, BMI, or serum levels of liver-associated enzymes.
| No. steatosis(n = 10) | Mild steatosis(n = 11) | Moderate-severe steatosis(n = 6) | Reference values | |
| Age | 36.3 ± 13.1 | 44.5 ± 14.4 | 48.3 ± 11.3 | N/A |
| Gender (% female) | 90% | 73% | 66% | N/A |
| Body mass index (kg/m2) | 42.4 ± 3.2 | 44.2 ± 6.2 | 41.9 ± 4.3 | 18.5-24.9 |
| Aspartate transaminase (U/L) | 28.8 ± 13.2 | 42.8 ± 34.2 | 47.0 ± 26.9 | 0-40 |
| Alanine transaminase (U/L) | 36.1 ± 18.7 | 50.1 ± 48.2 | 75.8 ± 40.1 | 0-45 |
| Alkaline phosphatase (IU/L) | 79.2 ± 18.0 | 89.5 ± 16.6 | 82.5 ± 12.7 | 20-140 |
Using the most common phenotypic definition of NKT cells, we sought to compare the percentage of CD3+/CD56+ in the liver and the periphery of subjects without hepatic steatosis with those with moderate-to-severe steatosis. As shown in Table 2, the liver and blood of subjects with steatosis had significant increases in the percentage of NKT cells. CD3+/CD56+ NKT cells comprised 38.6% ± 10.5% of all intrahepatic T cells of subjects with moderate-to-severe steatosis, compared to 21.5% ± 14.3% T cells in the liver of subjects without any steatosis (P = 0.03). The percentage of CD3+/CD56+ T cells in the liver of subjects with mild steatosis (24.1% ± 12.4%) was intermediate between that of normal and moderate-to-severe steatosis, and was significantly lower than that of the subjects with moderate-to-severe steatosis (P = 0.05) with a correlation of 0.93. While in all three subject cohorts, the percentage of CD3+/CD56+ cells was significantly lower in the blood compared to the liver, the percentage PBMC CD3+/CD56+ NKT cells of subjects with moderate-to-severe steatosis (12.3% ± 5.6%) was significantly greater than both subjects with no steatosis (5.1% ± 5.5%, P = 0.05) and those with mild steatosis (2.5% ± 1.5%, P = 0.02).
| Lymphocyte population | N steatosis | M steatosis | MS steatosis | P value(NvsM) | P value(NvsMS) | P value(MvsMS) | Correlationcoefficient |
| CD3+/CD4-/CD8- PBMC | 8.13 | 3.62 | 22.88 | 0.22 | 0.1 | 0.04a | 0.73 |
| CD3+/CD4-/CD8- IHL | 12.61 | 9.12 | 26.58 | 0.4 | 0.1 | 0.05a | 0.76 |
| CD3+/CD56+ PBMC | 5.09 | 2.45 | 12.32 | 0.22 | 0.049a | 0.016a | 0.71 |
| CD3+/CD56+ IHL | 21.49 | 24.13 | 38.62 | 0.7 | 0.03a | 0.048a | 0.93 |
| CD3+/CD56+/CD161+ PBMC | 2.45 | 1.15 | 9.64 | 0.3 | 0.027a | 0.017a | 0.79 |
| CD3+/CD56+/CD161+ IHL | 15.50 | 18.90 | 35.81 | 0.6 | 0.006a | 0.017a | 0.93 |
| CD3+/Vα24+ PBMC | 0.60 | 0.53 | 0.57 | 0.48 | 0.23 | 0.14 | -0.43 |
| CD3+/Vα24+ IHL | 0.43 | 0.42 | 0.76 | 0.9 | 0.37 | 0.36 | 0.85 |
| CD3+/CD8+ IHL | 55.59 | 49.30 | 26.58 | 0.51 | 0.0003a | 0.006a | -0.95 |
We also analyzed invariant NKT cells; the CD-1d-reactive, glycolipid-activating NKT cells which express vα24[7]. We found that a minority of CD3+/CD56+ NKT cells express vα24. In addition, we did not find significant differences in expression of vα24 between subjects without steatosis, those with mild or those with moderate-to-severe steatosis in the liver or blood (Table 2). When stimulated by alpha-galactosylceramide, the prototypical stimulant of vα24, invariant NKT cells, hepatic-derived lymphocytes produced greater amounts of IFN-γ, as measured by ELISA, compared to peripheral lymphocytes, though no differences were noted between patient cohorts (data not shown). In the majority of samples, IL-4 secretion remained undetectable.
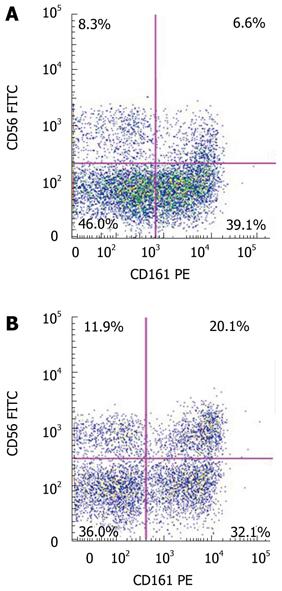
CD161 (NKR-P1A) is a receptor that is primarily associated with NK cells, but is also expressed on NKT cells, and may indicate an effector and memory subset of such cells[19]. We therefore assessed the expression of CD161 on the CD3+/CD56+ populations in the liver and blood (Figure 1). Again, in each cohort, there were a higher percentage of CD3+/CD56+ cells that expressed CD161 in the liver, compared to the blood (Table 2). Further, the percentage of CD161-expressing CD3+/CD56+ cells in the liver (35.8% ± 9.1%) and blood (9.6% ± 4.9%) of subjects with moderate-to-severe hepatic steatosis were significantly increased compared to those without steatosis (liver: 15.5% ± 12.6%, P = 0.01, blood: 2.5% ± 3.8%, P = 0.03) and those with mild hepatic steatosis (liver: 18.9% ± 12.5%, P = 0.02, blood: 1.2% ± 1.1%, P = 0.02).
In addition to increases in the percentages of NKT cells, other minor lymphocyte subsets were significantly affected in patients with moderate-to-severe hepatic steatosis. Intrahepatic percentages of double negative T cells (CD3+, CD4-, CD8-) were increased in the liver of subjects with moderate-severe steatosis (26.6% ± 17.0%), compared to those without steatosis [12.6% ± 10.4%, P = 0.05 (Table 2)].
The CD3+/CD8+ lymphocytes were the only lymphocyte population found to significantly decrease in patients with moderate-to-severe steatosis. In these patients, the percentage of CD3+/CD8+ lymphocytes (27.3% ± 9.6%) decreased significantly as compared to patients with mild steatosis (49.%3 ± 10.7%, P < 0.001) or without steatosis (55.6% ± 14.3%, P < 0.001). CD3+/CD8+ lymphocytes also decreased in the peripheral blood in patients with moderate-to-severe steatosis as compared to normal livers and approached significance (17.4% ± 8.5% vs 26.2% ± 7.0%, P = 0.06).
NAFLD and NASH are increasing in importance throughout the world. While our immune system plays an important role in the pathogenesis of this disease, our understanding of the specifics of the immunopathogenesis of NAFLD is limited. Much of our information regarding NAFLD has come from murine models, and NKT cells have been shown to be a key mediator of murine fatty liver disease[12]. However, there are very few studies of intrahepatic NKT cells in humans. In this study, we sought to investigate the changes in lymphocyte populations, with a focus on NKT cells, in obese patients with histologically confirmed steatosis or steatohepatitis. We found that NKT cells, defined as CD3+/CD56+ lymphocytes, are significantly increased in patients with moderate to severe steatosis as compared to patients with no steatosis or mild steatosis. These findings differ from the numerous studies performed in mice and suggest a different role of NKT cells in fatty liver disease in humans.
There have been 4 previous studies investigating NKT cells and fatty liver disease in humans, each using different techniques and yielding different results. In a study by Xu and colleagues, the investigators found a decrease in peripheral vα24+ NKT cells as compared to healthy matched non-obese controls[14]. In that study, the diagnosis of NAFLD was made on a clinical basis, as opposed to our utilization of histology, which is a more specific means of diagnosis, and IHLs were not examined. In a study by Kremer and colleagues, the investigators also found a decrease in NKT cells in patients with moderate to severe steatosis[15]. However, they defined NKT cells by expression of CD3+/CD57+, and used immunohistochemistry staining instead of flow cytometry for quantification, both of which can account for the differences in their results and ours. Finally, Tajiri and colleagues evaluated liver biopsy specimens of patients with NAFLD and performed flow cytometry on 20 of the specimens. In these 20 specimens, they found that in patients with more severe steatosis there was an increase in CD3+/CD56+ NKT cells[16], and is in agreement with the results reported here. Finally Syn et al[17] studied 6 liver biopsies, 2 of which had confirmed NASH cirrhosis, and found an increase percentage of NKT cells in the livers with NASH cirrhosis compared to healthy controls and patients with other forms of hepatitis. With 27 patients enrolled in this study, this is the largest sample size to date to evaluate lymphocyte populations in patients with NAFLD. Further, we also quantified the presence of invariant NKT cells, expression of CD161 and other minor T cell populations in our biologic samples, as well as examining cytokine production.
NKT cells may play a number of immunoregulatory roles in the liver and are considered by some to be a bridge between the innate and adaptive immune systems[20]. NKT cells participate in pro-inflammatory, Th1, and anti-inflammatory Th2 mediated pathways via the secretion of IFN-γ and IL-4, respectively. In murine models, it has been proposed that depletion of NKT cells shifts the hepatic immune environment toward a Th1 milieu, leading to immunocyte infiltration and development of steatohepatitis[9]. Leptin deficient mice develop steatosis and NASH, but they do not develop cirrhosis[20]. Alternatively, NKT cells, when shifting the immune environment toward a Th2 milieu may be responsible for collagen deposition in the liver. Stimulation and proliferation of NKT cells in leptin deficient mice, through adrenergic stimulation, results in hepatic collagen deposition and fibrosis secondary to IL-4 and IL-13 secretion and activation of Th2 mediated pathways[12,20]. In our study, we found an increased percentage of intrahepatic CD3+/CD56+ NKT cells in patients with moderate to severe steatosis and a low incidence of steatohepatitis, which could support a protective role of NKT cells against steatohepatitis. In addition we found a decrease in CD3+/CD8+ intrahepatic lymphocytes which may implicate NKT cells in shifting the hepatic immunoregulatory environment towards more Th2 mediated mechanisms. We were unable to identify a difference in the secretion of IFN-γ or IL-4 by NKT cells in patients with various degrees of steatosis, although interferon, but not IL-4 production was elaborated when NKT cells were stimulated in the liver samples studied. Future studies should focus on investigating the functional role of NKT cells in human fatty liver disease.
The multiple definitions of NKT cells can lead to much confusion when discussing their role in the liver. We classified NKT cells in two different ways, both by expression of CD3+/CD56+, as well as by expression of vα24+. Human NKT cells were initially described in liver donor patients by Doherty et al[21] as CD3+/CD56+ cells and were shown to be capable of lysing NK sensitive cells. CD3+/CD56+ lymphocytes have been analyzed for mRNA expression of vα24 and approximately 5% of human hepatic CD3+/CD56+ lymphocytes expressed vα24 mRNA, which encodes the TCR that recognizes CD1d ligands[21]. Thus, NKT cells are also defined functionally as vα24+ lymphocytes or via isolation of CD3+ lymphocytes using CD1d ligands, and are classified as invariant NKT cells. The CD3+/CD56+ lymphocytes, which are also called NKT-like cells, are populations that incorporate many different type of lymphocytes such as invariant T-cells and CD161+ lymphocytes which can potentially create confusion[22,23]. Thus, although we find that this broader more diverse population (CD3+/CD56+ NKT cells) is significantly increased with greater degrees of steatosis, the more specific subgroup of invariant vα24 NKT cells were unchanged. It is possible that other functional subgroups of CD3+/CD56+ lymphocytes such as CD161+ lymphocytes play a larger role in human NAFLD and NASH. This is in contrast to the murine model where there are higher percentages of invariant NKT cells normally found in the liver[24]. These findings highlight the importance of investigating the role of invariant NKT and NKT-like lymphocytes in human disease, rather than just using murine models.
The results of the study are limited by the small sample size, and impaired our ability to further characterize the role of NKT cells in NAFLD. Nevertheless the increase in NKT cells in moderate to severe steatosis was significant and correlates with other studies. Absolute lymphocyte numbers were not reported here because the values were affected by the varied size of the liver biopsy samples taken in each patient. Thus, NKT cell percentages of total lymphocyte were reported for more precise comparison between subjects. Immunohistochemistry has not yet been performed on the liver biopsies, however we hope to conduct future studies to further elucidate the role of NKT cells in NAFLD.
In this study, we examined the change in lymphocyte populations in obese patients with NAFLD, with a focus on intrahepatic NKT cells. We found an increase in NKT cells, defined as CD3+/CD56+ and as well as CD161+ lymphocytes, in obese patients with moderate and severe steatosis. These results differ from previous murine models and some human studies. In addition we reported other changes in lymphocyte populations, such as depletion in CD3+/CD8+ lymphocytes and an increase in CD3+/CD4-/CD8- cells, which have not yet been reported in NAFLD. The results of this study highlight the importance of investigating NKT cells and other lymphocyte populations in humans with NAFLD since the pathophysiology of human NAFLD likely differs from that in murine models. Future studies to investigate the role of NKT cells in NAFLD in humans are warranted in order to elucidate the mechanisms behind the pervasive disease of NAFLD.
Non alcoholic fatty liver disease (NAFLD) is a common disease where fat infiltrates the liver, which can lead to inflammation and cirrhosis. natural killer T (NKT) cells have been implicated in the pathogenesis of NAFLD. In obese mice, NKT cells are depleted in the liver and are associated with a greater degree of steatosis. When the NKT cells are upregulated in mice, the degree of fatty infiltration diminishes. There is limited data about NKT cells in human NAFLD, and this study adds to our understanding of NKT cells in human NAFLD.
The data on intrahepatic NKT cells and its role in human steatosis has been mixed. There have been 3 studies investigating NKT cells and NAFLD. One found that NKT cells are depleted with increased steatosis whereas the others found an increase in NKT cells with steatosis. This study contains the largest sample to date which investigates NKT cells in human NAFLD.
The authors found that NKT cells, defined as CD3+/CD56+ lymphocytes are increased in human livers with moderate and severe steatosis. In addition, they reported other changes in lymphocyte populations with steatosis, such as depletion in CD3+/CD8+ lymphocytes and an increase in CD3+/CD4-/CD8- cells, which have not yet been reported in NAFLD.
These findings further support the role of NKT cells in NAFLD and highlight an important difference between NKT cells in the murine model of fatty liver disease and human NAFLD.
NKT cells are a highly conserved subset of lymphocytes with properties of both T cells (CD3+ expression) and NK cells (CD56+ and CD161+ expression). The liver contains a high percentage of these unique lymphocytes, which have been implicated in the pathogenesis of non alcoholic fatty liver disease.
The authors showed that patients with moderate or severe steatosis had a higher percentage of intrahepatic CD3+/CD56+ NKT cells than that with mild steatosis or without steatosis. Further, the percentage of CD3+/CD56+CD161+ cells in the liver of subjects with moderate-to-severe hepatic steatosis were significantly increased compared to those with mild hepatic steatosis or without steatosis. This is an interesting finding and may provide more information about the NKT cells in human NAFLD because the data on NKT cells in human NAFLD is limited at present. However, there are several areas of the manuscript that the authors should expand upon that would enhance the presentation.
Peer reviewer: Dongchang Zhao, Professor of Beckman Research Institute, Department of Surgery, 1500 Duarte Road, Duarte, CA 91010, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S17-S29. [Cited in This Article: ] |
| 2. | Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486. [Cited in This Article: ] |
| 3. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [Cited in This Article: ] |
| 4. | Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102:1351-1356. [Cited in This Article: ] |
| 5. | Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573-583. [Cited in This Article: ] |
| 6. | Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520-525. [Cited in This Article: ] |
| 7. | Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180:3627-3635. [Cited in This Article: ] |
| 8. | Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304-1310. [Cited in This Article: ] |
| 9. | McClure N, Henry O, Harley JM. Pure XY gonadal dysgenesis presenting as secondary amenorrhea. A case report. J Reprod Med. 1992;37:291-292. [Cited in This Article: ] |
| 10. | Shiratori Y, Kawase T, Komatsu Y, Hikiba Y, Okano K, Kamii K, Omata M. Endotoxin induced cellular communication in the liver: murine models for clarification of the role of LPS-responsive macrophages in the pathogenesis of liver diseases. J Gastroenterol Hepatol. 1995;10 Suppl 1:S97-S100. [Cited in This Article: ] |
| 11. | Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, Alper R, Thalenfeld B, Engelhardt D, Rabbani E. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121-128. [Cited in This Article: ] |
| 12. | Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, Thomas SA, Diehl AM. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434-441. [Cited in This Article: ] |
| 13. | Margalit M, Shalev Z, Pappo O, Sklair-Levy M, Alper R, Gomori M, Engelhardt D, Rabbani E, Ilan Y. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther. 2006;319:105-110. [Cited in This Article: ] |
| 14. | Xu CF, Yu CH, Li YM, Xu L, Du J, Shen Z. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:4504-4508. [Cited in This Article: ] |
| 15. | Kremer M, Thomas E, Milton RJ, Perry AW, van Rooijen N, Wheeler MD, Zacks S, Fried M, Rippe RA, Hines IN. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51:130-141. [Cited in This Article: ] |
| 16. | Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:673-680. [Cited in This Article: ] |
| 17. | Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998-2007. [Cited in This Article: ] |
| 18. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [Cited in This Article: ] |
| 19. | Diehl AM. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1-G5. [Cited in This Article: ] |
| 20. | Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. 2006;176:211-216. [Cited in This Article: ] |
| 21. | Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314-2321. [Cited in This Article: ] |
| 22. | Choi S, Diehl AM. Role of inflammation in nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2005;21:702-707. [Cited in This Article: ] |
| 23. | Peralbo E, Alonso C, Solana R. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp Gerontol. 2007;42:703-708. [Cited in This Article: ] |
| 24. | Exley MA, Koziel MJ. To be or not to be NKT: natural killer T cells in the liver. Hepatology. 2004;40:1033-1040. [Cited in This Article: ] |