Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.971
Revised: November 17, 2011
Accepted: December 31, 2011
Published online: March 7, 2012
AIM: To investigate the expression of B7-H1 in human colorectal carcinoma (CRC) to define its regulating effects on T cells in tumor microenvironment.
METHODS: One hundred and two paraffin blocks and 33 fresh samples of CRC tissues were subject to this study. Immunohistochemistry was performed for B7-H1 and CD3 staining in CRC tissues. Ficoll-Hypaque density gradient centrifugation was used to isolate peripheral blood mononuclear cells of fresh CRC tissues; flow cytometry and immunofluorescence staining were used for detection of regulatory T cells. Data was analyzed with statistical software.
RESULTS: Costimulatory molecule B7-H1 was found strongly expressed in CRC tissues, localized in tumor cell membrane and cytoplasm, while weak or none expression of B7-H1 was detected in pared normal colorectal tissues. Meanwhile, CD3 positive T cells were found congregated in CRC tumor nest and stroma. Statistic analysis showed that B7-H1 expression level was negatively correlated to the total T cell density in tumor nest (P < 0.0001) and tumor stroma (P = 0.0200) of 102 cases of CRC tissues. Among the total T cells, a variable amount of regulatory T cells with a clear Foxp3+ (forkhead box P3) staining could be detected in CRC tissues and patients’ blood. Interestingly, in the 33 samples (15 cases of B7-H1high CRC tissues and 18 cases of B7-H1low CRC tissues) of freshly isolated mononuclear cells from CRC tissues, the percentages of CD4+Foxp3+ and CD8+Foxp3+ regulatory T cells were found remarkably higher in B7-H1high CRC tissues than in B7-H1low CRC tissues (P = 0.0024, P = 0.0182), indicating that B7-H1 expression was involved in proliferation of regulatory T cell. No significant difference was found in CRC peripheral blood (P = 0.0863, P = 0.0678). PD-1 is the specific ligand for B7-H1 pathway transferring inhibitory signal to T cell, which is expressed by activated T cell. Our further analysis of PD-1 expression on T cells in CRC tissues showed that conventional T cells (CD4+Foxp3-/CD8+Foxp3-), which was thought to contribute to the anti-tumor immune response, highly expressed PD-1; while regulatory T cells (CD4+Foxp3+/CD8+Foxp3-) almost failed to express PD-1. The average percentage of PD-1 expression on regulatory T cells was significantly higher than the percentage of PD-1 on conventional T cells (CD4+Foxp3- T cell, P < 0.0001; CD8+Foxp3- T cell, P < 0.0001). The diverse expression of PD-1 might lead to different fate of T cell subsets in B7-H1 over-expression CRC tumor microenvironment.
CONCLUSION: B7-H1 expression in tumor cells can inhibit the conventional T cell proliferation in tumor microenvironment through the PD-1 expression on conventional T cells.
- Citation: Hua D, Sun J, Mao Y, Chen LJ, Wu YY, Zhang XG. B7-H1 expression is associated with expansion of regulatory T cells in colorectal carcinoma. World J Gastroenterol 2012; 18(9): 971-978
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/971.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.971
Tumor genesis is associated with a wide array of both genetic and epigenetic changes. Although host immune surveillance may prevent tumor outgrowth during the earliest stages of tumor growth, locally invasive or metastatic tumors must evade host immunity[1]. Immune escape is not merely a passive process of immune evasion but an active process in which tumor cells, stromal cells and immune cells within the tumor microenvironment actively suppress the antitumor immune response. Both myeloid-derived cells and lymphocyte subsets, most notably regulatory T cells (Treg), collaborate with their malignant counterparts to suppress the host immunity[2,3]. Recent evidence showed that experimental depletion of Tregs improves immune-mediated tumor clearance and enhances the response to immune-based therapy[4,5]. Tregs have been shown to suppress tumor-specific T cell immunity and therefore may contribute to the progression of human tumors[6,7]. Furthermore, tumor Tregs are associated with a reduced survival of the patients with ovarian carcinoma and brain tumors[8,9]. In contrast, it has been found in Hodgkin lymphoma that decreased number of infiltrating Foxp3+ cells in conjunction with increased infiltration of cytotoxic T lymphocytes predicts an unfavorable clinical outcome[10]. Although previous studies have suggested that tumors could induce CD25+Foxp3+Tregs from naïve CD4 T cells in the absence of thymus, the cellular and molecular mechanisms for that, however, are still not well understood[11,12]. Optimal activation of antigen-specific lymphocytes requires specific antigen recognition by lymphocytes and costimulatory signals[13]. Up to date, a cohort of important costimulatory molecules, including B7 family ligands, and those which interact with known or unknown receptors, has been identified, namely B7-1 (CD80), B7-2 (CD86), B7-H1 (PD-L1), B7-DC (PD-L2), B7-H2 (ICOS ligand), B7-H3 and B7-H4 (B7x, B7-S1), which essentially contribute to the T cell activation and tolerance[14-19]. B7-H1 was identified in 1999 as a member of B7 family that was described to negatively regulate T cell function by engagement with PD-1, a CD28 family member receptor. Besides antigen-presenting cells, B7-H1 mRNA was found in a variety of nonlymphoid parenchymal organs, including the heart, placenta, skeletal muscle, and lung[15]. B7-H1 was thought to inhibit T cell growth and cytokine production by ligation of the PD-1 receptor[20], which is expressed on activated T and B cells[15,21]. Zou et al[22] reported the presence of B7-H1 protein by immunohistochemistry in a wide range of human cancers. Tumor-associated B7-H1 induced apoptosis of effector T cells and was thought to contribute to immune evasion by cancer. Other studies indicated that blockade of B7-H1 enhanced tumor immunity but had no direct effect on tumor cells[23-25]. To make the situation more complicated, B7-H1 can also function as a receptor to transmit signals to T cells and tumor cells[26,27]. In summary, B7-H1 can act as both ligand and receptor to execute immuno-regulatory functions. B7-H1 was reported to be involved in the induction of Tregs, dysfunctional dendritic cells that expressed up-regulated B7-H1 may lead to the generation of Tregs, and in vivo blocking of B7-H1 signaling abolished the conversion in a tumor-induced Treg conversion model[28]. Whether the tumor-associated B7-H1 could affect the Tregs generation in the tumor microenvironment deserves further exploration.
Colorectal carcinoma (CRC) is one of the most frequent malignancies worldwide, its incidence and mortality are especially high in Western developed counties, and it is the second leading cause of cancer-related death[29]. CRC is a multi-pathway disease since numerous pathological factors and polygene transformation are involved in its oncogenesis and progression. Within recent decades, varieties of therapeutic strategies including conventional surgery, chemotherapy, radiotherapy and immunotherapy, or even combination of these therapies have been available in the treatment of CRC patients. However, these therapies yielded different outcomes due to different physical conditions of the patients, which shaped the tumor microenvironment with immune suppressions[30-32]. Therefore, it is critical for clinicians to perform further analysis of the immune suppression and establish individualized strategy for CRC patients.
In the present study, we performed immunohistochemistry to characterize the B7-H1 expression in human CRC and examined its effect on infiltrating T lymphocytes in tumor tissues. The regulatory T cells were detected in the tumor tissues and peripheral blood of CRC patients, and the relationship between the B7-H1 expression and Tregs population was analyzed. The mechanism of regulatory T cell expansion related to B7-H1-PD-1 signal was also investigated.
For B7-H1 expression and T cell infiltration analysis, 102 patients with CRC who underwent surgery from May 2004 to December 2007 were included in the present study. No patient received pre-operative chemotherapy or radiotherapy. The paraffin blocks of tumor tissues were assembled from the archival collections of the Department of Pathology, and all 102 specimens were identified as CRC by hematoxylin and eosin (HE) staining. For regulatory T cell and B7-H1 expression analysis, 33 CRC patients who underwent surgery from January 2008 to July 2009 were subjected to this study. No patient received pre-operative chemotherapy or radiotherapy. The freshly removed tumor tissues were identified as colorectal carcinoma by pathologist according to the HE staining. The blood samples were collected from the 33 CRC patients before the surgery by venipuncture. Thirty-three normal tissues from autologous non-malignant portion of colon or rectum were resected surgically for the analysis as well, and used as the normal tissue control.
The specimens were collected from the Third Affiliated Hospital and the Fourth Affiliated Hospital of Soochow University, China, with the approval of the Ethic Committees of these hospitals.
Immunohistochemistry was performed using the Dako ElivisionTM according to the manufacturer’s instructions. Both tumor tissues and non-malignant tissues were fixed with formalin, and embedded in paraffin wax. Before immunohistochemical staining, 3-μm-thick consecutive sections were cut by microtome, dewaxed in xylene and rehydrated through graded ethanol solutions. Antigens were retrieved by heating the tissue sections at 100 °C for 30 min with citrate solution (10 mmol/L, pH 6.0, for CD3 antigen retrieval) or ethylenediaminetetraacetic acid solution (1 mmol/L, pH 8.0, for B7-H3 antigen retrieval). Sections were cooled down and immersed in 0.3% hydrogen peroxide for 15 min to block endogenous peroxidase activity, and then rinsed in phosphate-buffered saline (PBS) for 5 min, blocked with 5% bovine serum albumin (BSA) at room temperature for 15 min, and incubated with primary antibodies against CD3 (Maixin Biotechnology Limited Corporation, Fuzhou, China) and B7-H1[21], respectively, at 4 °C overnight. Negative controls were performed by replacing the specific primary antibody with PBS. The sections were then rinsed in PBS for 5 min for 3 times and were followed by incubation with HRP-labeled goat anti mouse/rabbit secondary antibody (Dako, Glostrup, Denmark). Diaminobenzene was used as the chromogen and hematoxylin as the nuclear counterstain. The sections were dehydrated, cleared and mounted.
Two independent observers who blinded to the clinicopathological parameters of the patients assessed the immunohistochemical staining sections. The B7-H1 immunostaining intensities were scored according to a scale as grade 0, negative; grade 1, weakly positive; grade 2, moderately positive; grade 3, strongly positive. The negative means no tumor cells showing positive immunostaining. Sections were considered as positive when the tumor cells showed cytoplasmic or membranous B7-H1 immunostaining with proper intensities and were extended as grades 1, 2 and 3. The sections of grade 0 and grade 1 were classified as low expression group, and other sections of grade 2 and grade 3 were classified as high expression group.
Infiltrating T lymphocytes in both tumor stroma and tumor nest were determined according to the CD3 immunolabeling. First, the infiltrating T lymphocytes in tumor stroma were examined at low magnification (× 40) and categorized according to the density as: grade 0, scanty; grade 1, moderate infiltration; grade 2, abundant infiltration; grade 3, the most abundant infiltration. The group of grade 0 and grade 1 was defined as low infiltration group, and another group of grade 2 and grade 3 was defined as high infiltration group. Second, the infiltrating T lymphocytes in tumor nest were counted as follows: five areas in tumor nest with the most intense infiltrating T lymphocytes were selected at low magnification (× 40), then the infiltrating T lymphocytes were counted and recorded at high power field (HPF, × 200 magnification). Results from the five areas were averaged and used in the statistical analysis. In the present study, the sections with the infiltrating T lymphocytes in tumor nest of less than 60 per HPF were defined as low infiltration group, and other sections with the infiltrating T lymphocytes in tumor nest of more than 60 per HPF were defined as high infiltration group. The cutoff point of 60 T lymphocytes per HPF for low/high infiltration assessment in tumor nest was set at the median value of the entire sections.
Fresh tumor specimens were gently minced on a wire mesh screen to obtain a cell suspension. The cell suspension was centrifuged over Ficoll-Hypaque (Amersham Biosciences, Sweden) at 1400 r/min for 25 min. After density gradient centrifugation, the mononuclear cells were collected and washed with RPMI 1640 media (Gibco, United States) containing 50 g/mL fetal bovine serum (Hyclone, United States) and 10 g/mL penicillin/streptomycin (Sigma-Aldrich, United States). Peripheral blood mononuclear cells were also isolated with Ficoll-Hypaque density gradient centrifugation, and the isolated mononuclear cells were subjected to the analysis immediately.
Cells were washed in PBS containing 5 g/mL BSA and incubated with the specific fluorochrome-conjugated antibodies identifying surface molecules on T cells for 30 min at 4 °C. The antibodies included CD4-FITC, CD8-FITC (Beckman Coulter, United States) and PD-1-PE (eBioscience, United States). For intracellular staining, washed cells were fixed with Foxp3 Fixation/Permeabilization Solution (eBioscince, United States) at room temperature, then incubated with PE-cy5 conjugated anti-Foxp3 antibody (eBioscince, United States) at room temperature in the dark for 30 min. Labeled cells were re-suspended in 0.5 mL cell staining buffer, and then were analyzed with flow cytometry and the Beckman-Coulter’s Expo32 Multicomp software (Beckman Coulter, United States). Isotype controls were done for each staining.
Statistical analyses were performed using GraphPad Prism 5.0 software package (GraphPad Software, United States). Paired or unpaired Student’s t test, Wilcoxon signed rank test, and the Pearson χ2 test were used where appropriate. A P value less than 0.05 was considered statistically significant.
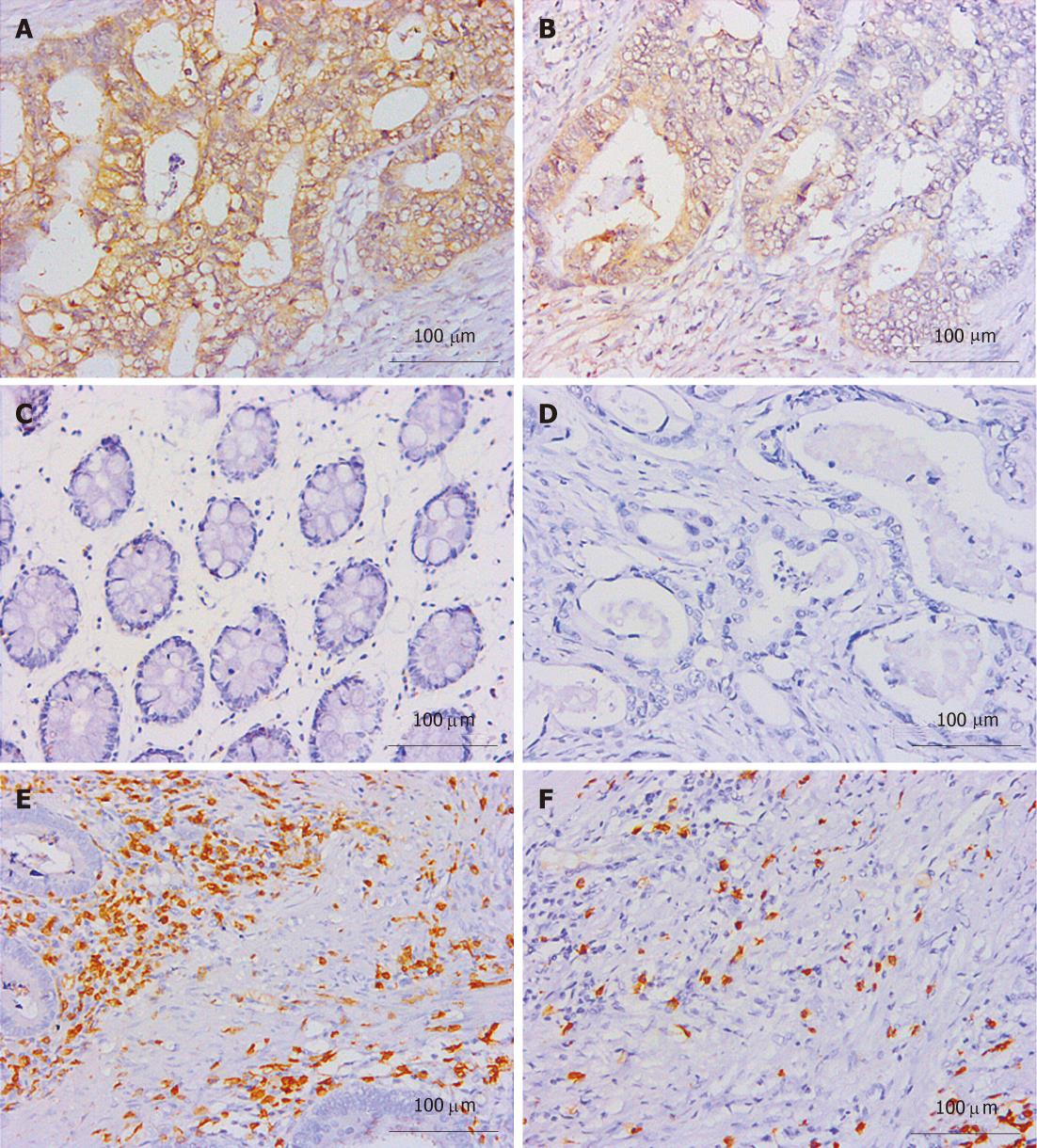
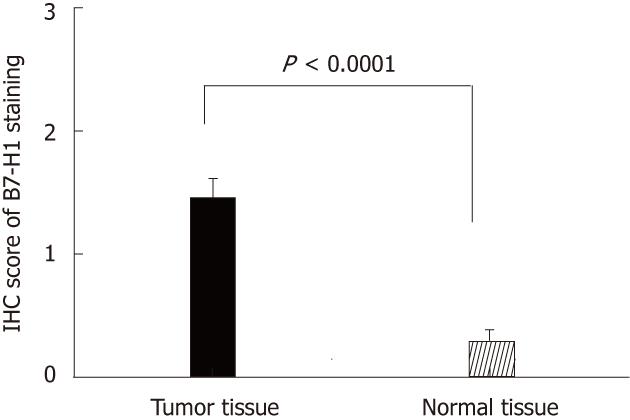
Thirty-three cases of paired CRC tissues and adjacent normal tissues resected surgically were used to investigate the B7-H1 expression. As we described in previous works in human gastric carcinoma[21], B7-H1 was also found strongly expressed in tumor cells, localized in the membrane and cytoplasm (Figure 1A and B). Based on the immunohistochemical scores, 33 cases of tumor tissues showed B7-H1 expression (Score 1, 2 or 3), and 15 cases of tumor tissues had high B7-H1 expression (Score 2 or 3). In the normal tissues, 3 cases showed low B7-H1 expression (Score 1), and none showed high B7-H1 expression (Figure 1C). According to the statistical analysis, B7-H1 expression level in CRC tumor tissues was higher than in the adjacent normal colorectal tissues (P < 0.0001, Figure 2).
CD3 staining was considered as T cell labeling, and the number of positive cells represented the total T cell infiltration density[30]. CD3 positive T cells were found congregated in CRC tumor and stroma (Figure 1E and F). One hundred and two cases of paraffin embedded CRC tissues were used to study the B7-H1 expression and the T cell infiltration. As shown in Table 1, tumor cell B7-H1 expression was negatively and significantly correlated to the density of CD3 positive T cells in tumor nest (P < 0.0001, Table 1) and tumor stroma (P = 0.0200, Table 1). Thus, the data further implied an important role of B7-H1 in suppressing T cell-based cellular immune surveillance of CRC.
| Infiltrating T lymphocytes in colorectal carcinoma tissues | Cases | B7-H1 expression | χ2 | P value | |
| Low | High | ||||
| Tumor stroma | 9.549 | 0.0200 | |||
| Low infiltration | 68 | 24 | 44 | ||
| (scanty and moderate) | |||||
| High infiltration | 34 | 23 | 11 | ||
| (abundant and the most abundant) | |||||
| Tumor nest | 17.4 | < 0.0001 | |||
| Low infiltration (less than 60 T lymphocytes per HPF) | 51 | 13 | 38 | ||
| High infiltration (more than 60 T lymphocytes per HPF) | 51 | 34 | 17 | ||
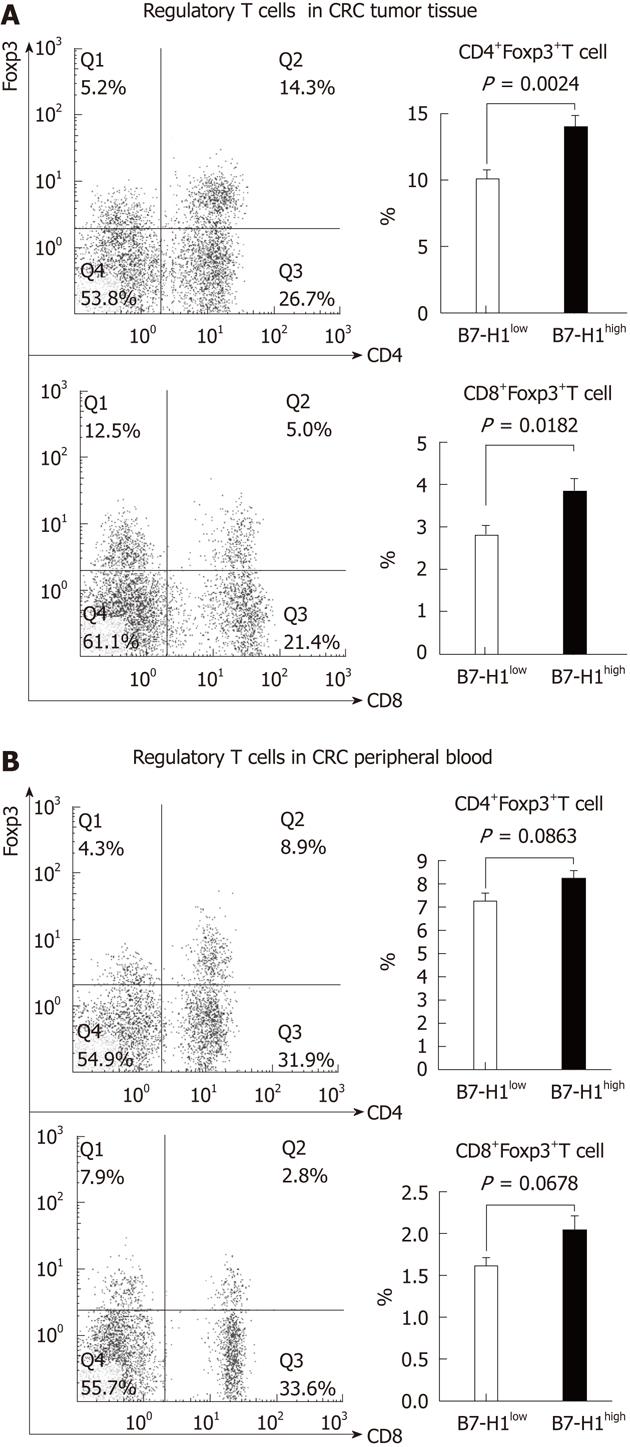
To evaluate the effect of tumor B7-H1 on intratumoral regulatory T cells, we first characterized the percentage of CD4+Foxp3+ T cells and CD8+Foxp3+ T cells. Mononuclear cells isolated from resected specimens of CRC patients were stained with multicolor-labeled antibodies and analyzed by flow cytometry. The peripheral blood mononuclear cells were also analyzed in the regulatory cell population. Among the CD4+ or CD8+ T cells, a population with a clear Foxp3+ could be detected (Figure 3). A variable amount of regulatory T cells was found in CRC tissues and patients’ blood. Next, to determine the correlation between the tumor cell B7-H1 expression and regulatory T cells, we divided the 33 CRC specimens into B7-H1high group (n = 15) and B7-H1low group (n = 18) according the B7-H1 expression in tumor tissues. The results showed that in the CRC tumor tissues, the percentages of CD4+Foxp3+ T cells and CD8+Foxp3+ T cells in B7-H1high group were remarkably higher than in the B7-H1low group (P = 0.0024, P = 0.0182). On the contrary, in the CRC peripheral blood, there was no significant difference of the percentages of regulatory T cells between B7-H1high group and B7-H1low group (P = 0.0863, P = 0.0678).
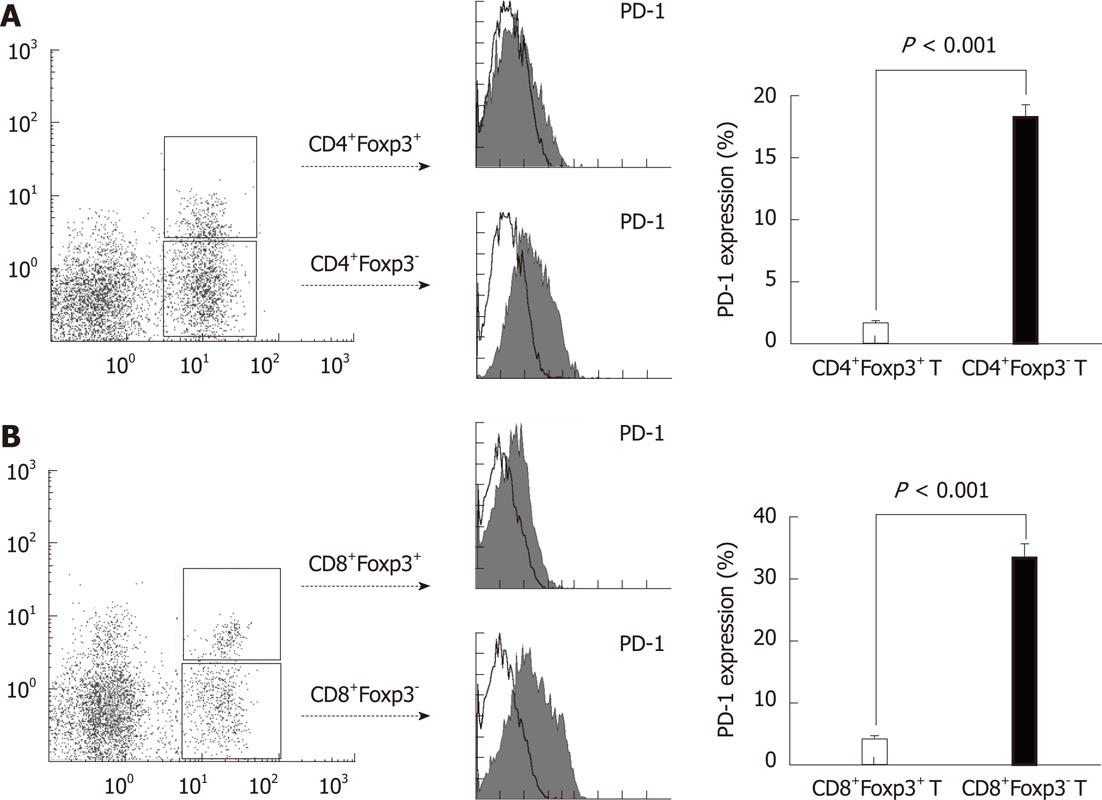
PD-1-B7-H1 ligation has been shown to have an inhibitory effect on T cells[33]. However, the effect of this pathway on regulatory T cells infiltrated in tumor tissues has not been investigated. The highly expressed B7-H1 on tumor cells could lead to a low amount of total T cell infiltration, but a high amount of regulatory T cell infiltration in the local tissues, which indicated that the PD-1-B7-H1 pathway could be different in the conventional T cells and regulatory T cells. Therefore, we assessed the surface PD-1 expression on CD4+Foxp3+/CD8+Foxp3+ regulatory T cells and CD4+Foxp3-/CD8+Foxp3- conventional T cells in CRC tissues. We found a high percentage of CD4+Foxp3-/CD8+Foxp3- conventional T cells expressing PD-1 on the cell surface (Figure 4, P < 0.0001). However, the CD4+Foxp3+/CD8+Foxp3+ regulatory T cells could hardly express PD-1 (Figure 4, P < 0.0001), which could allow the survival of regulatory T cells in B7-H1high tumor microenvironment.
B7-H1, with engagement of either of its receptor, PD-1, plays a critical role in suppressing T cell-based immunity and has emerged as an important mediator of tumor-associated immune suppression. B7-H1 was found in several solid tumors and evaluated as a potent prognostic factor. Our previous work has described the immunosuppressive effects of B7-H1 pathway in gastric carcinoma[33]. We demonstrated in this study that B7-H1 was over-expressed in human colorectal carcinoma, whereas B7-H1 was not expressed or expressed at a very low level in normal colorectal tissues. Furthermore, we found that the protein levels of B7-H1 in tumor cells were negatively correlated to the densities of total T cells, suggesting that it may encompass a mechanism of immune suppression in antitumor immunity in human CRC. This part of work is consistent with the previous studies of B7-H1 in other solid tumors, which again emphasize the important role of up-regulated B7-H1 expression in human tumors. Tumors have a specific microenvironment that contains many types of immune cells. Although the potent value of B7-H1 in tumor progression was confirmed repeatedly, the mechanism of tumor-associated B7-H1 involved in the regulation of these immune cells is still poorly understood.
Regulatory T cells have been identified in the lung, gastric and esophageal, ovarian, breast and pancreatic tumor specimens, and Hodgkin lymphoma. It has been suggested that CD4+CD25+Treg cells are involved in the mediation of antitumor immunity by suppressing tumor-specific T cell immunity, thereby contributing to the growth of human tumors[6-10]. How regulatory T cells could survive and expand in the tumor microenvironment remains unclear, and how to regulate the regulatory T cells in microenvironment would be critical to tumor immunotherapy[34]. In the present study, we detected the CD4+Foxp3+ and CD8+Foxp3+ regulatory T cell population in the CRC tumor tissues and peripheral blood. The percentage of regulatory T cells was significantly elevated in B7-H1high CRC tissues, but not in the peripheral blood. These results indicated that the up-regulated B7-H1 expression could lead to a local congregation of regulatory T cells, which might promote the tumor progression. B7-H1 expression was positively related to the regulatory T cell expansion, but negatively related the total T cell infiltration in CRC tissues. This suggested that B7-H1 pathway could have different effects and functions on the subgroups of T cells. B7-H1 pathway could participate in the immune suppressions through multi-regulations of T cells.
T cells are a very diverse lymphocyte population with respective functions in tumor microenvironment. Understanding of their polarization toward stimulatory or inhibitory activity is important to know how they work in diseases[30,35]. Regulatory T cells expressing the hallmark forkhead transcription factor 3 (Foxp3) are of therapeutic value in cancer immunotherapy due to their potent immunosuppressive effects. The presence of regulatory T cells was determined by the complex tumor microenvironment. How to decrease the number of regulatory T cells and increase the conventional T cells is critical to obtain a desired outcome of immunotherapy. Here, we assessed the PD-1 expression on T cell subsets to explore the mechanism of B7-H1 pathway in regulation of tumor-infiltrated T cells. The data showed a very interesting phenomenon that conventional T cells (CD4+Foxp3- /CD8+Foxp3-) expressed PD-1 on the cell surface at a high level, while regulatory T cells (CD4+Foxp3+ /CD8+Foxp3+) almost failed to express PD-1. Under these circumstances, the ligation of tumor-associated B7-H1 with PD-1 on conventional T cells would lead to the failure of T cell-mediated anti-tumor effect, and inhibit the conventional T cell proliferation and survival. At the same time, the loss of PD-1 expression could allow the existence and expansion of regulatory T cells, which could further inhibit the conventional T cells. B7-H1 expression in tumor cells could collaborate with its regulation outcomes on T cells, suppress the tumor immune response, and shape the tumor immune escape circumstance.
In conclusion, the inhibitory co-stimulatory molecule B7-H1 expression was found in CRC tissues, but not in normal colorectal tissues. B7-H1 expression in tumor cells could inhibit the conventional T cell proliferation in tumor microenvironment through the PD-1 expression on conventional T cells. Regulatory T cells could barely express PD-1, and consequently gain the survival and expansion in a B7-H1high microenvironment. Our works also support the efforts to develop immunotherapeutic approaches targeting B7-H1 pathway for the treatment of CRC.
We thank the senior pathologist Tan Y from the Third Affiliated Hospital of Soochow University for his professional suggestions and technical assistances. We also thank Professor Jiang JT from the Third Affiliated Hospital of Soochow University, Dr. Jiang D and Dr. Chen Y from the First Affiliated Hospital of Soochow University for their instructions in the collection of experimental specimens.
Inhibitory co-stimulatory molecules from B7-family have been implicated in suppression of tumor immunity. B7-H1 was found to be over-expressed in many human malignancies, and was significantly correlated to the clinicopathological parameters and prognoses of various human tumors. B7-H1 was suggested to play an important role in tumor immune escape, while the full mechanism of tumor-associated B7-H1 pathway needs further studies.
B7-H1 expression in tumor tissues was evaluated as a potent target for tumor immune therapy. The mechanism of B7-H1 pathway was considered involving the suppressive effect on tumor-specific T cell immunity and therefore may contribute to the progression of human tumors. Previous studies have suggested that tumors could induce CD25+Foxp3+ regulatory T cells (Treg) from naïve CD4 T cells in the absence of thymus, while the key molecules and pathway are still unknown. In this study, the authors focus on the significance and function of tumor-associated B7-H1 in Tregs’ expansion.
The mechanism of B7-H1 pathway in tumor microenvironment is commonly considered as decreasing and suppressing of antigen-specific T cells. This article reported for the first time that tumor-associated B7-H1 was involved in the expansion of Tregs in colorectal carcinoma (CRC) tissues, which provided a new strategy for the future researches. Furthermore, B7-H1-PD-1 was found not responsible for the Tregs expansion, which offers a presumption of another receptor for B7-H1 on Tregs.
Regulatory T cells are of theraputic value in cancer immunotherapy due to their potent immunosuppressive effects. How to decrease the number of regulatory T cells and increase the conventional T cells is critical to obtain a desired outcome of immunotherapy. This study supports the efforts to develop immunotherapeutic approaches targeting B7-H1 pathway for immune therapy through regulating conventional and regulatory T cells in CRC.
The authors investigated the immunolocalization of the protein B7-H1 in colorectal cancer tissues using a standardized immunohistochemical approa- ch. Furthermore, they compared the B7-H1 expression with the density of FOXP3 and CD3 immune cell infiltrate. They found that B7-H1 expression in tumor cells inhibits the proliferation of T cells, but allows the survival and expansion of T regulatory lymphocytes. The manuscript is interesting, and well done.
Peer reviewers: Fabio Grizzi, PhD, Laboratories of Quantitative Medicine, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, Rozzano 20089, Milan, Italy; Theodora Choli-Papadopoulou, Associate Professor, Department of Biochmeistry, School of Chemistry, Aristotle University of Thessaloniki, Thessaloniki 55124, Greece
S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L
| 1. | Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807-839. [PubMed] [Cited in This Article: ] |
| 2. | Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267-296. [PubMed] [Cited in This Article: ] |
| 3. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [PubMed] [Cited in This Article: ] |
| 4. | Li X, Kostareli E, Suffner J, Garbi N, Hämmerling GJ. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol. 2010;40:3325-3335. [PubMed] [Cited in This Article: ] |
| 5. | Poehlein CH, Haley DP, Walker EB, Fox BA. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol. 2009;39:3121-3133. [PubMed] [Cited in This Article: ] |
| 6. | Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277-288. [PubMed] [Cited in This Article: ] |
| 7. | Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591-2601. [PubMed] [Cited in This Article: ] |
| 8. | Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, Adema GJ. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225:195-199. [PubMed] [Cited in This Article: ] |
| 9. | Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-949. [PubMed] [Cited in This Article: ] |
| 10. | Alvaro T, Lejeune M, Salvadó MT, Bosch R, García JF, Jaén J, Banham AH, Roncador G, Montalbán C, Piris MA. Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467-1473. [PubMed] [Cited in This Article: ] |
| 11. | Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628-636. [PubMed] [Cited in This Article: ] |
| 12. | Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488-4495. [PubMed] [Cited in This Article: ] |
| 13. | Markman M. PET/CT scans in ovarian cancer: prognostic versus predictive utility? Minerva Med. 2009;100:415-420. [PubMed] [Cited in This Article: ] |
| 14. | Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515-548. [PubMed] [Cited in This Article: ] |
| 15. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [PubMed] [Cited in This Article: ] |
| 16. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [PubMed] [Cited in This Article: ] |
| 17. | Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827-832. [PubMed] [Cited in This Article: ] |
| 18. | Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269-274. [PubMed] [Cited in This Article: ] |
| 19. | Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849-861. [PubMed] [Cited in This Article: ] |
| 20. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] [Cited in This Article: ] |
| 21. | Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765-772. [PubMed] [Cited in This Article: ] |
| 22. | Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467-477. [PubMed] [Cited in This Article: ] |
| 23. | Lin PY, Sun L, Thibodeaux SR, Ludwig SM, Vadlamudi RK, Hurez VJ, Bahar R, Kious MJ, Livi CB, Wall SR. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J Immunol. 2010;185:2747-2753. [PubMed] [Cited in This Article: ] |
| 24. | Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067-8075. [PubMed] [Cited in This Article: ] |
| 25. | Pulko V, Liu X, Krco CJ, Harris KJ, Frigola X, Kwon ED, Dong H. TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol. 2009;183:3634-3641. [PubMed] [Cited in This Article: ] |
| 26. | Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CL, Chen L. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111:363-370. [PubMed] [Cited in This Article: ] |
| 27. | Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635-3643. [PubMed] [Cited in This Article: ] |
| 28. | Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331-9336. [PubMed] [Cited in This Article: ] |
| 29. | Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153-165. [PubMed] [Cited in This Article: ] |
| 30. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [PubMed] [Cited in This Article: ] |
| 31. | Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668-676. [PubMed] [Cited in This Article: ] |
| 32. | Watanabe K, Nagai K, Kobayashi A, Sugito M, Saito N. Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg. 2009;96:1058-1065. [PubMed] [Cited in This Article: ] |
| 33. | Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y, Chen Y, Shi Q, Yu G, Zhang X. PD-L1 expression analysis in gastric carcinoma tissue and blocking of tumor-associated PD-L1 signaling by two functional monoclonal antibodies. Tissue Antigens. 2007;69:19-27. [PubMed] [Cited in This Article: ] |
| 34. | Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. [PubMed] [Cited in This Article: ] |