Article Text
Abstract
Background: CD40 has been shown to be a functional activation antigen on a variety of cell types involved in immune responses. As intestinal fibroblasts and myofibroblasts may play a role during mucosal inflammation, we investigated the functional consequences of CD40 induction in primary cultures of human colonic fibroblasts.
Methods: Primary colonic lamina propria fibroblasts (PCLF) were isolated from endoscopic biopsies and surgical specimens. Cultures were used between passages 3 and 9. CD40 surface display was determined by FACS analysis and mRNA expression by reverse transcription-polymerase chain reaction. Secretion of cytokines was determined by ELISA. Nuclear factor κB (NFκB) activation was shown by electrophoretic mobility shift assay (EMSA).
Results: After priming with interferon γ (IFN-γ) (200 U/ml) for 72 hours, five of eight tested PCLF cultures showed induction of CD40 surface display (up to 10-fold). Induction of CD40 mRNA expression was demonstrated by semiquantitative polymerase chain reaction. In the responder-PCLF cultures, IFN-γ alone caused a 1.5–5-fold increase in interleukin (IL)-8 secretion. Addition of 1 ng/ml CD40L was sufficient to achieve a further increase in IL-8, IL-6, or monocyte chemotactic protein 1 (MCP-1) secretion (2.5–18-fold of controls). Incubation with CD40L alone without priming with IFN-γ had no effect. The proteasome inhibitor N-acetyl-leucinyl-leucinyl-norleucinal (ALLN 100 µM) reduced IFN-γ/CD40L mediated cytokine induction, suggesting participation of NFκB, which was directly demonstrated by EMSA. CD4+ T cells induced MCP-1 secretion by PCLF, which was prevented by addition of an excess of CD40-IgG fusion protein. CD40 expression on PCLF could also be demonstrated in vivo by immunohistochemistry.
Conclusion: The CD40-CD40L pathway augments mucosal inflammatory responses via mucosal PCLF. CD40-CD40L mediated T cell/PCLF interactions could play an important role during intestinal mucosal inflammation.
- CD40
- CD40L
- myofibroblasts
- cytokines
- inflammatory bowel disease
- nuclear factor κB
- ALLN, N-acetyl-leucinyl-leucinyl-norleucinal
- BSA, bovine serum albumin
- CD, cluster of differentiation
- DAB, diaminobenzidine
- DMEM, Dulbecco’s modified Eagle’s medium
- EMSA, electrophoretic mobility shift assay
- FCS, fetal calf serum
- HBSS, Hank’s buffered salt solution
- IBD, inflammatory bowel disease
- IEC, intestinal epithelial cells
- IFN, interferon
- IL, interleukin
- ICAM-1, intercellular adhesion molecule 1
- LPMNC, lamina propria mononuclear cells
- MCP-1, monocyte chemotactic protein 1
- NFκB, nuclear factor κB
- PBS, phosphate buffered saline
- PCLFs, primary human colonic lamina propria fibroblasts
- TNF, tumour necrosis factor
- TRAF, TNF receptor associated factor
Statistics from Altmetric.com
- ALLN, N-acetyl-leucinyl-leucinyl-norleucinal
- BSA, bovine serum albumin
- CD, cluster of differentiation
- DAB, diaminobenzidine
- DMEM, Dulbecco’s modified Eagle’s medium
- EMSA, electrophoretic mobility shift assay
- FCS, fetal calf serum
- HBSS, Hank’s buffered salt solution
- IBD, inflammatory bowel disease
- IEC, intestinal epithelial cells
- IFN, interferon
- IL, interleukin
- ICAM-1, intercellular adhesion molecule 1
- LPMNC, lamina propria mononuclear cells
- MCP-1, monocyte chemotactic protein 1
- NFκB, nuclear factor κB
- PBS, phosphate buffered saline
- PCLFs, primary human colonic lamina propria fibroblasts
- TNF, tumour necrosis factor
- TRAF, TNF receptor associated factor
Mesenchymal cells such as fibroblasts, myofibroblasts, or smooth muscle cells produce a number of mediators known to be important effectors during inflammation. Skin, synovial, and pulmonary mesenchymal cells can secrete growth factors such as granulocyte colony stimulating factor and granulocyte macrophage-colony stimulating factor,1 interleukins (IL) such as IL-1, IL-6, or IL-8,2–6 or chemokines such as RANTES or monocyte chemotactic protein 1 (MCP-1).7 These mediators have been shown to play an important role in the pathogenesis of inflammatory bowel disease (IBD) (for a review see Rogler and Andus8). Cytokine and chemokine secretion is upregulated by bacterial lipopolysaccharide5,9–11 or by contact with T cells,12 suggesting a role of mesenchymal cells during host defence. Hitherto only few studies have investigated the role of intestinal mesenchymal cells as active participants in mucosal inflammation and the pathogenesis of IBD.
CD40, a 45–50 kDa membrane glycoprotein of 277 amino acids, was originally described as a functionally important B cell surface molecule.13–15 The ligand for CD40, CD40L, is a 33–39D glycoprotein of 261 amino acids, which is a member of the tumour necrosis factor (TNF) superfamily.15–17 In recent years it has become clear that CD40 is not only expressed by B cells but by many other cell types such as antigen presenting cells,18–20 endothelial cells, epithelial cells from different organs, and fibroblasts.21–25 CD40L is produced as a type II transmembrane protein and may be present on the cell surface as a heteromultimeric complex, composed of membrane bound and soluble forms.26–28 Apart from its 33 kDa form two shorter versions of the protein (31 kDa and 18 kDa) exist as soluble proteins.29,30 The receptor, CD40, activates several second messenger systems, including protein-tyrosine kinases, phosphatidylinositol 3-kinase, phospholipase Cγ2, and serine-threonine stress activated protein kinases.31,32 These different pathways finally result in activation of transcription factors such as nuclear factor κB (NFκB) or NF-AT.31,32
Several proteins were shown to interact with the intracellular domain of CD40. The first molecule identified was CD40 binding protein (CD40bp) now termed TRAF3 (TNF receptor associated factor 3).33–36 Besides TRAF3, CD40 associates with TRAF237 as well as TRAF538 and TRAF6.39,40
Ligation of CD40 by CD40L may be involved in several autoimmune diseases and, interestingly, CD40L antibodies abrogated experimental colitis in mice.41–43 Recent data also demonstrated a functional role of the CD40-CD40L system on mesenchymal cells (for example, human synovial and lung fibroblasts and airway smooth muscle cells).22,24,25,27,44,45 Fibroblast activation by the CD40/CD40L system could be increased by pretreatment of cells with interferon γ (IFN-γ).24,46 Recently, we demonstrated that colonic fibroblasts secrete a row of inflammatory cytokines but the mechanisms involved in the regulation of cytokine secretion by these cells have still to be examined.9
CD40 has been suggested to play a role in IBD pathophysiology. Liu et al found increased expression of CD40L on freshly isolated lamina propria T cells from IBD patients compared with controls. They identified increased numbers of CD40+ and CD40L+ cells in IBD mucosa, being B cells, macrophages, and CD4+ T cells, respectively.47 In peripheral blood of Crohn’s disease patients, increased numbers of CD40+ monocytes and CD40+ dendritic cells were found.48,49 The pathophysiological role of the CD40/CD40L system in IBD was further supported by the immunohistochemical demonstration of increased numbers of CD40+ cells in ulcerative colitis mucosa50 and by the finding of increased blood levels of CD40L, particularly in Crohn’s disease.51
These data indicate that the CD40-CD40L system may be of relevance for secretion of inflammatory mediators by intestinal fibroblasts during the pathogenesis of IBD. We therefore addressed this question in vitro using cultures of isolated primary human colonic lamina propria fibroblasts (PCLFs) and by immunohistochemistry.
MATERIAL AND METHODS
Patients
PCLF cultures were isolated from either endoscopic biopsies or surgical specimens. Cultures from eight patients without macroscopic or histological signs of inflammation (mean age 47.2 (17.2) years) were used. The study was approved by the University of Regensburg Ethics Committee.
Isolation of intestinal fibroblasts
In the case of surgical specimens, mucosa was stripped from submucosa, minced in 1 mm pieces, and placed in Dulbecco’s modified Eagle’s medium (DMEM) (PAA, Cölbe, Germany) with 20% fetal calf serum (FCS) (Gibco, Karlsruhe, Germany). Endoscopic biopsies were placed in sterile DMEM with 10% FCS and PenStrep (Gibco, Karlsruhe, Germany). Epithelial cells were removed and lamina propria fibroblasts isolated and cultured as described previously.9 Cells were used between passages 3 and 9.
Immunohistochemistry
Characterisation of colonic PCLF
The following antibodies were used for characterisation of PCLF: vimentin (Clone V9; Abcam, France), antifibroblast antibody ASO2 (Dianova, Hamburg, Germany), α-smooth muscle cell actin (Clone 1A4; Dako, Hamburg, Germany), CD45 (Clone HI30; Pharmingen, San Diego, California, USA), EP4 (Clone Ber-EP4; Dako), cytokeratin 18 (Clone DC10; Coulter-Immunotech, Krefeld, Germany), CD68 (Clone KP1; Dako), CD3 (Clone UCHT1; Coulter-Immunotech), antihuman fibroblast surface protein (Clone 1B10; Sigma, Taufkirchen, Germany), isotype control IgG1 (MOPC 21; Sigma), isotype control IgG2 (MOPC-141; Sigma), isotype control IgG3 (FLOPC-21; Sigma).
Cells were seeded onto LabTek Chamber slides (Nunc, Wiesbaden, Germany). After fixation with 3.7% formaldehyde, endogenous peroxidase was inactivated by incubation with 0.3% H2O2 for 30 minutes. Blocking was done with 1% bovine serum albumin (BSA) for 30 minutes. Cells were incubated with the primary antibody for one hour and with a biotin-SP conjugated rabbit antimouse IgG antibody (Dianova, Baltimore, Pennsylvania, USA) for 30 minutes followed by the avidin biotin complex (Zymed, San Francisco, California, USA) for one hour (all at room temperature). Binding of antibodies was visualised with 0.03% 3-3′ diaminobenzidine (DAB) (Sigma, St Louis, Missouri, USA) and 0.003% hydrogen peroxide until a brown reaction product could be seen.
Immunohistochemical double staining
For double labelling of freeze sections, additional anti-CD40 (clone MAB89; Coulter) and anti-intercellular adhesion molecule 1 (ICAM-1; Dianova) were used. Tissue sections were fixed in 3.7% buffered formaldehyde followed by acetone (50%, 100%, 50%; two minutes each) and rehydrated in 0.1 M phosphate buffered saline (PBS) followed by 1% BSA for blocking. Incubation with anti-CD40 was performed overnight at 4°C. Incubation with the biotinylated secondary antibody (Dianova) was performed at room temperature for one hour. Staining with DAB was done as described for PCLF cultures.
For double staining, the remaining peroxidase was suppressed by incubation in 0.3% hydrogen peroxide for 30 minutes. Sections were then incubated with the second incubation series consisting of primary and secondary antibodies and avidin biotin complex, as described above. For colour reaction, the specimen was incubated according to the VectorVIP (purple) (Vector Laboratories, Burlingame, California, USA) protocol producing an intense purple coloured precipitate.
Stimulation experiments with PCLF
Cells were seeded into sixwell plates (Falcon, Heidelberg, Germany) at a density of 2–5×105 per well. Cells were grown to confluence before stimulation experiments to ensure comparable conditions, with the majority of cells in the G0 phase of the cell cycle.
Cultured PCLF were stimulated for the indicated periods. N-acetyl-leucinyl-leucinyl-norleucinal (ALLN) 100 µM was added to the medium in parallel for inhibition of NFκB activation. After the incubation period, medium was removed for determination of secreted proteins and cells were harvested with 1% sodium dodecyl sulphate in PBS for protein assay.
Determination of cytokine protein
Cytokines were measured by ELISA (Biotrak-Amersham, Braunschweig, Germany or R&D systems, Wiesbaden, Germany) according to the manufacturer’s protocol.
FACS analysis of CD40 expression on PCLF
Flow cytometry was performed using an EPICS flow cytometer (Coulter, Miami, USA) equipped with an argon ion laser with an excitation power of 15 mW at 488 nm. Cells were incubated with FITC labelled anti-CD40 antibody or isotype control for two hours at 4°C in the dark. Acquisition was performed on unfixed cells. For data analysis, WIN-MDI software was used.
Reverse transcription-polymerase chain reaction (RT-PCR) for CD40, CD40L, and TRAF3 mRNA
Total mRNA from cell cultures was prepared using RNeasy columns (Qiagen, Hilden, Germany). Reverse transcription was performed with the reverse transcription system of Promega (Mannheim, Germany). A 900 bp fragment of the CD40 cDNA was amplified using the 5′ primer aatctagatgccgcctggtctcacctcg and the 3′ primer aaaagcttgccaactgcctgtttgcccacg. A 630 bp fragment of the CD40L cDNA was amplified using the 5′ primer cggccactggactgcccatcagc and the 3′ primer gcccgcaaggtttggcggaactg. A 300 bp fragment of the TRAF3 cDNA (CD40bp) was amplified using the 5′ primer cctgcgagaccacgtggagaaggcg and the 3′ primer gcaggttgacgtgctgcacggcgg. The PCR conditions were 1×PCR buffer, 200 µmol/l dNTPs, 0.5 µmol/l primer, 0.5 U Taq polymerase, and 0.125 µg cDNA for each PCR. PCR conditions were: 94°C, five minutes; 94°C, 30 seconds; 68°C, one minute, 72°C, 45 seconds.
Isolation of LPMNC, CD33+ macrophages, and CD4+ T cells
Isolation of lamina propria mononuclear cells (LPMNC) and CD33 positive intestinal macrophages was performed essentially as described previously.52 Isolated LPMNCs were sorted by immunomagnetic beads coated with monoclonal antibodies against CD33 (intestinal macrophages) or CD4 (T helper cells) (MACS Beads, Miltenyi Biotec, Bergisch Gladbach, Germany) with a separation column and a magnetic separator from the same company.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts from PCLF were prepared as described previously.53 The prototypic double stranded immunoglobulin kappa chain oligonucleotide (5′-CAGAGGGACTTT CCGAGA-3′) was used as a probe and labelled by annealing of complementary primers followed by primer extension with the Klenow fragment of DNA polymerase I (Boehringer Mannheim, Mannheim, Germany) in the presence of [α-32P]dCTP (>3.0 Ci/mmol; Amersham, Arlington Heights, Illinois, USA) and deoxynucleoside triphosphates I (Boehringer Mannheim, Mannheim, Germany) as described previously.53 Nuclear extracts (5 µg protein) were incubated with radiolabelled DNA probes (≈10 ng, 105 cpm) for 30 minutes at room temperature.53 Samples were run in 0.25× TBE buffer non-denaturing 6% polyacrylamide gels at 125 V for three hours. Equal amounts of protein were loaded onto the gels. Gels were analysed by autoradiography.
Statistics
Unless stated otherwise, all data are given as mean (SD). Statistical analysis was performed using the Student’s t test for unpaired samples or the Mann-Whitney rank sum test as indicated.
RESULTS
IFN-γ induced CD40 expression on PCLF
Eight different PCLF cultures isolated from non-inflamed mucosa of control patients were used for the experiments. PCLF cultures were characterised with the antibody panel given in the methods section to exclude contamination with other cell types. All cultures were positive and homogeneously stained with antifibroblast antibody ASO2 as well as anti-vimentin. α-Actin was positive in up to 40% of cells (range 6–40%), indicating that PCLF cultures were not homogeneous. Homogeneity was not increased by passaging. After the third passage, CD45, CD3, CD68, or EP4 were positive in less than 1% of cultured cells, indicating the absence of lymphocytes, macrophages, or epithelial cells.
For induction of CD40 expression, PCLF cultures were primed with 200 U/ml IFN-γ for 72 hours. Higher concentrations or longer incubation periods did not further increase CD40 surface expression. Lower concentration of IFN-γ or shorter incubation periods caused lower numbers of CD40 positive cells in FACS analysis (data not shown).
In FACS analysis, five of eight primary PCLF cultures showed induction of CD40 expression after priming with IFN-γ (fig 1A). Mean fluorescence was increased up to 10-fold compared with control conditions. In two cultures only part of the PCLF population showed induction of CD40: two separate populations were observed with obvious difference in CD40 surface expression (fig 1B). The five cultures which showed CD40 expression after IFN-γ stimulation were termed “responder PCLF cultures”.
FACS analysis of interferon γ (IFN-γ) induced CD40 expression on colonic primary human colonic lamina propria fibroblasts (PCLFs). Histogram of FITC-anti-CD40 fluorescence on colonic PCLFs with and without IFN-γ preincubation (200 U/ml, 72 hours). IFN-γ induced an up to 10-fold increase in FITC fluorescence (filled graph) compared with uninduced cells (open graph) (A) in five of eight cultures. In two of the cultures not all cells showed induction of CD40 expression, indicated by two peaks in the histogram ((B); IFN-γ primed cells: filled graph).
RT-PCR confirmed induction of CD40 mRNA by IFN-γ in the responder PCLF cultures (fig 2A). Whereas no CD40 mRNA was detectable in unprimed PCLFs, incubation with 200 U/ml IFN-γ led to the appearance of the expected amplicon. For confirmation, the PCR product was cloned and sequenced. For comparison, CD40 mRNA expression was also determined in lymphocytes and freshly isolated macrophages. A positive signal was observed in the lymphocyte fraction (CD33 negative LPMNC) whereas freshly isolated intestinal macrophages (CD33+) from normal mucosa were negative for CD40 mRNA (fig 2).
Reverse transcription-polymerase chain reaction (RT-PCR) for CD40, CD40 ligand (CD40L), and tumour necrosis factor receptor associated factor (TRAF3) expression in primary human colonic lamina propria fibroblasts (PCLFs) with and without priming with interferon γ (IFN-γ). RT-PCR for CD40 (900 bp fragment), for CD40L (630 bp fragment), TRAF3 (300 bp fragment), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH 450 bp fragment) was performed and analysed on 1% agarose gels (n = non-stimulated, p = IFN-γ primed). Three independent experiments are shown. IFN-γ induced CD40 whereas CD40L could not be amplified and the TRAF3 signal was not consistently influenced. The lymphocyte fraction (CD33 negative lamina propria mononuclear cells (LPMC)) and freshly isolated intestinal macrophages (CD33+) were used for comparison. The results are representative of three or more experiments.
CD40L mRNA was absent in PCLF and could not be induced with IFN-γ (fig 2). However, CD40L mRNA was clearly expressed in the lamina propria lymphocyte population (CD33 negative LPMNC). Isolated colonic macrophages did not show CD40L mRNA, indicating that mainly lymphocytes express CD40L mRNA in the normal human intestinal mucosa (fig 2). TRAF3 (CD40bp) was constitutively expressed in all cells investigated (fig 2). These data indicate that CD40 mRNA expression is upregulated by IFN-γ in PCLF and that CD40L mRNA is absent.
Induction of cytokine secretion by CD40 ligation
To investigate whether induction of CD40 protein expression on PCLF is followed by cell reactivity to soluble CD40L, we incubated for 72 hours IFN-γ primed and unprimed PCLF cultures with 1, 10, or 50 ng/ml CD40L. In the responder PCLF cultures, IFN-γ alone caused a 1.5–5-fold increase in IL-8 secretion (fig 3A). Addition of 1 ng/ml CD40L was sufficient to achieve a further increase in cytokine secretion (2.5–18 fold of control) (fig 3B). Incubation with CD40L alone without prior IFN-γ priming had no effect. Very similar results were found for IL-6 (fig 3C, D) and MCP-1 (fig 3E, F). The CD40L effect on cytokine secretion of PCLF after priming with IFN-γ was strongest with respect to IL-6 compared with IL-8 and MCP-1. Taken together, these results indicate that CD40L stimulation of responder PCLF enhanced cytokine secretion.
Induction of cytokine secretion in primary human colonic lamina propria fibroblasts (PCLFs) with and without priming with interferon γ (IFN-γ). IFN-γ primed (200 U/ml for 72 hours) and unprimed PCLF were incubated with 1, 10, or 50 ng/ml CD40L. (A), (C) and (E) show typical “responder” cultures whereas (B), (D), and (F) show the mean (SD), as percentage of controls, indicated number of cultures. (A) and (B) give data for interleukin (IL)-8 secretion, (C) and (D) for IL-6, and (E) and (F) for monocyte chemotactic protein 1 (MCP-1). Cytokines were measured in culture supernatants by ELISA.
Activation of NFκB by CD40 ligation
As NFκB is involved in CD40 signalling in other cell types and is a potent regulator of IL-8, IL-6, or MCP-1 secretion, we investigated the possible role of NFκB for CD40 signalling in PCLF. The proteasome inhibitor ALLN (100 µM) was tested for toxicity by trypan blue exclusion and propidium iodide cell cycle analysis. No evidence of toxic effects during the incubation period was found (data not shown). As shown in fig 4, ALLN alone reduced spontaneous MCP-1 secretion by PCLF, indicating basic activation of NFκB. Priming with IFN-γ increased MCP-1 secretion. Addition of CD40L to unprimed cells had no effect on cytokine secretion whereas it doubled MCP-1 secretion in IFN-γ primed cells. Addition of ALLN abolished the stimulating effect of CD40L on cytokine secretion of primed cells. This indicated that the proteasome inhibitor ALLN was able to antagonise the effect of CD40L on primed human PCLFs (fig 4).
Effect of the protease inhibitor N-acetyl-leucinyl-leucinyl-norleucinal (ALLN) on monocyte chemotactic protein 1 (MCP-1) induction by CD40L. Interferon γ (IFN-γ) primed (200 U/ml for 72 hours) primary human colonic lamina propria fibroblasts were incubated with CD40L (50 ng/ml) and/or the proteasome inhibitor ALLN (100 µM). MCP-1 secretion in medium supernatants was determined by ELISA. Data are representative of two independent experiments.
To show that this effect was mediated by inhibition of NFκB activation, EMSA were performed (fig 5). Basal activation of NFκB was found in non-stimulated cells (fig 5A, lane 1). Addition of 1 ng/ml CD40L or 50 ng/ml CD40L strongly increased NFκB activation if cells had been preincubated with IFN-γ (fig 5A, lanes 2 and 3). In contrast, incubation of unprimed PCLF with 1 or 50 ng/ml CD40L did not activate NFκB (fig 5A, lanes 4 and 5). Addition of ALLN did not change the NFκB signal of untreated cells significantly (fig 5B, lanes 1 and 2). Priming with IFN-γ was not followed by significant NFκB activation if ALLN was present (fig 5B, lane 3). Moreover, ALLN also prevented CD40L induced NFκB activation seen without addition of ALLN (fig 5B, lane 4).
Effect of CD40L on nuclear factor κB (NFκB) activation in interferon γ (IFN-γ) primed primary human colonic lamina propria fibroblasts (PCLFs). Electrophoretic mobility shift assay (EMSA) of cultured PCLF stimulated as indicated. (A) Baseline NFκB translocation in controls is seen (lane 1). After priming with IFN-γ incubation with 1 ng/ml (lane 2) or 50 ng/ml (lane 3), CD40L was followed by NFκB activation. Without priming, 1 ng/ml (lane 4) or 50 ng/ml CD40L (lane 5) did not cause significant NFκB activation. These data are representative of two independent experiments. (B) Baseline activation of NFκB is seen in lane 1. Addition of N-acetyl-leucinyl-leucinyl-norleucinal (ALLN) did not change the NFκB signal of unprimed cells significantly (lane 2). Priming with IFN-γ was not followed by significant NFκB activation in the presence of 100 µM ALLN (lane 3). ALLN 100 µM also prevented CD40L induced NFκB activation (lane 4).
Effect of CD4+ T cells on PCLF cytokine secretion
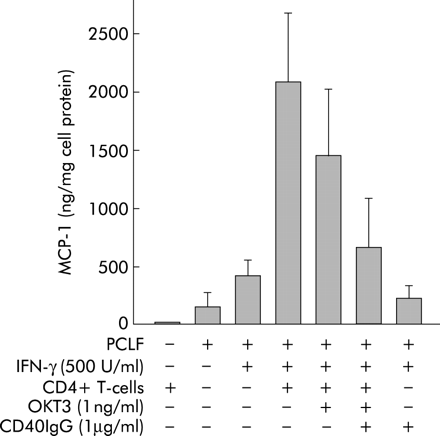
To test the functional relevance of upregulation of CD40 on PCLF by IFN-γ, we incubated responder PCLF cell cultures with freshly isolated peripheral blood CD4+ cells of different donors and measured MCP-1 secretion. The presence of CD40L on these cells was monitored by flow cytometry (data not shown). As indicated in fig 6, CD4+ cells did not produce MCP-1. PCLF showed basal secretion of MCP-1 which was further stimulated by priming with IFN-γ (fig 6). Incubation of primed PCLF with CD4+ T cells was followed by clear stimulation of MCP-1 secretion to about 500% of the secretion induced by IFN-γ alone. The neutralising anti-CD3 antibody OKT3 (1 ng/ml) had no significant effect on MCP-1 secretion of PCLF in coculture with CD4+ T cells. However, addition of CD40IgG fusion protein (1 µg/ml), which is thought to bind to CD40L present on CD4+ cells and inhibit the interaction of CD40L with CD40, reduced MCP-1 secretion almost to levels found with IFN-γ priming alone. This reduction was highly significant compared with IFN-γ+CD4 cells (p = 0.0004; rank sum test; power of test with alpha = 0.0500: 0.9947). It was also significant compared with IFN-γ+CD4 cells+OKT3 (p = 0.02; rank sum test). However, in this case the power of the test with alpha = 0.0500 was only 0.6184. These results indicate that T cells can stimulate PCLF to secrete chemokines or cytokines via CD40/CD40L interaction.
Effect of CD4+ cells and CD40IgG fusion protein on monocyte chemotactic protein 1 (MCP-1) secretion by primary human colonic lamina propria fibroblasts (PCLFs). Four “responder” PCLF cultures were incubated with freshly isolated peripheral blood CD4+ cells from two different donors each. MCP-1 secretion was measured by ELISA. PCLF showed basal secretion of MCP-1 which was further stimulated by priming with interferon γ (IFN-γ). Incubation of primed PCLF with CD4+ T cells was followed by induction of MCP-1 secretion. Addition of neutralising antibody against CD3 (OKT3) reduced MCP-1 secretion, but not significantly. Addition of CD40IgG fusion protein (1 µg/ml) reduced MCP-1 secretion significantly compared with IFN-γ+CD4 cells and IFN-γ+CD4 cells+OKT3.
Demonstration of CD40 induction on PCLF in vivo
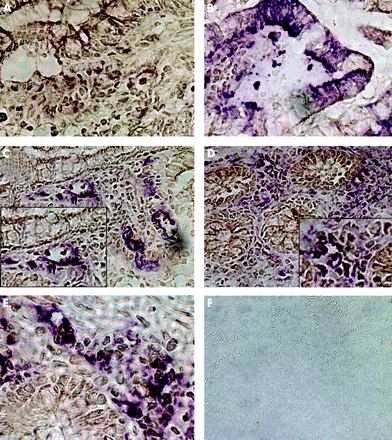
To investigate whether the in vitro data are relevant in vivo in IBD, we performed an immunohistochemical double staining for CD40, PCLF markers, EP4 as an epithelial cell marker, CD3 as a T cell marker, and ICAM-1 on five specimens in each group. In normal mucosa of biopsies of healthy donors, hardly any CD40 positive cells could be detected (brown reaction product, fig 7A). No colocalisation was found with the epithelial cell marker EP4 (fig 7B) or with the fibroblast markers ASO2 (fig 7C), vimentin (fig 7D), or 1B10 (fig 7E). Isotype controls were negative (fig 7F).
Immunohistochemical colocalisation of CD40 and fibroblast markers in normal mucosa. CD40 was visualised in all specimen by diaminobenzidine (brown reaction product). The indicated markers were stained in a second step with Vector violet (violet reaction product). (A) Almost absence of CD40 positive cells. No colocalisation with the epithelial cell marker EP4 (B) or with the fibroblast markers ASO2 (C), vimentin (D), or 1B10 (E). Isotype controls were negative (F).
In biopsies from patients with active Crohn’s disease, a strong increase in CD40 staining (always brown reaction product) was observed (fig 8A). Whereas staining on epithelial cells was mainly unspecific and no colocalisation of EP4 and CD40 could be detected (fig 8B), CD40 was colocalised with ICAM-1, indicating expression on endothelial cells (purple reaction product, fig 8C). Vimentin (purple, fig 8D) and ASO2 (purple, fig 8E) positive cells double stained for CD40, demonstrating the presence of CD40 on mesenchymal cells.
Immunohistochemical colocalisation of CD40 and fibroblast markers in normal mucosa. CD40 was visualised in all specimen by diaminobenzidine (brown reaction product). The indicated markers were stained in a second step with Vector violet (violet reaction product). (A) Almost absence of CD40 positive cells. No colocalisation with the epithelial cell marker EP4 (B) or with the fibroblast markers ASO2 (C), vimentin (D), or 1B10 (E). Isotype controls were negative (F).
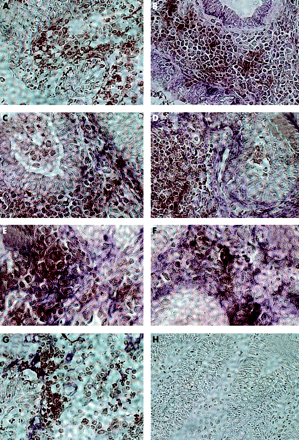
Similar results were found in mucosa from patients with ulcerative colitis (fig 9). The number of CD40 positive cells in the lamina propria was clearly increased (always brown reaction product, fig 9A). Whereas CD40 was not expressed in epithelial cells (EP4, purple; fig 9B) some of the CD40 positive cells could be identified as T cells (fig 9C). CD40 display could be colocalised with anti-α smooth muscle cells actin (fig 9D), ASO2 fibroblast marker (fig 9E), vimentin (fig 9F), or to a lesser extent with 1B10 (fig 9G). Therefore, it may be concluded that induction of CD40 on colonic PCLF also occurs during active ulcerative colitis. No specific staining was observed with the isotype control (fig 9H).
Immunohistochemical colocalisation of CD40 and fibroblast markers in active ulcerative colitis. (A) Increased number of CD40 positive cells in the lamina propria compared with control mucosa. No colocalisation with epithelial cells (EP4, (B)). Some of the CD40+ could be identified as T cells (C). Colocalisation with anti-α smooth muscle cells actin (D), ASO2 fibroblast marker (E), vimentin (F), and 1B10 (G), indicating CD40 induction on primary human colonic lamina propria fibroblasts during active ulcerative colitis. Lack of specific staining with isotype controls (H).
DISCUSSION
The hypothesis of the present study was that the CD40-CD40L system might be of relevance for secretion of inflammatory mediators of intestinal PCLF, particularly in the pathogenesis of IBD.
Our data indicate that IFN-γ induces expression of CD40 on primary human PCLF. Ligation of CD40 on these cells by CD40L is followed by NFκB activation and subsequent secretion of NFκB dependent cytokines. Soluble CD40L can stimulate cytokine secretion by PCLF but also the interaction of CD4+ T cells expressing CD40L with human PCLF can induce cytokine secretion from these cells. This is a new mechanism of cell-cell interaction of intestinal mesenchymal cells with T cells that may be responsible for induction of cytokine secretion during mucosal inflammation. In IBD, display of CD40 on colonic PCLF occurs in vivo, indicating that this mechanism could be of pathophysiological importance. The potential role of CD40 expression on mesenchymal cells is also supported by the study of Sempowski et al who reported similar results for human orbital fibroblasts.25 These cells also were activated via CD40, and CD40 expression was upregulated by IFN-γ.25
In the gut, investigations on the role of CD40 ligation have focused on the function of macrophages and T cells.47 Here we show that non-classical immune cells such as PCLF can also play an important role in cell-cell interactions during intestinal inflammation. CD40L positive T cells not only activate cytokine secretion in macrophages but also in PCLF. As the normal resting mucosal macrophage displays an inactive “anerg” phenotype, it has been speculated that this cell might not be initially activated and may not be the starting point of the inflammatory cascade. Thus could intestinal PCLF be the trigger for intestinal inflammation?
It is important to note that CD40 expression on PCLF has to be induced by IFN-γ. As IFN-γ is a major T cell product during a Th-1 inflammatory response, it is obvious that an inflammatory reaction has to occur before PCLF can be activated. However, activated Th-1 cells in the vicinity of PCLF may induce CD40 expression on PCLFs and consecutively stimulate PCLF to secrete cytokines further activating T cells or macrophages inducing cell invasion into the inflamed tissue and a self perpetuating type of chronic inflammation.
It is not clear why not all cultures of PCLF could be primed by IFN-γ to express CD40. Immunocytochemical characterisation of cultures revealed no obvious differences. Incubation with IFN-γ alone, without ligation of CD40, induced cytokine secretion and NFκB activation after 72 hours. As there is no known direct activation of NFκB by IFN-γ, it is likely that apart from CD40, IFN-γ induces another cytokine or mediator which is responsible for NFκB activation.
Among the molecules induced by CD40 ligation on PCLF are IL-8, which can attract neutrophils, MCP-1, which attracts monocytes/macrophages, and ICAM-1. It has been shown previously that activation of intestinal fibroblasts induces ICAM-1 surface expression and mRNA as well as adhesiveness for T cells.54 ICAM-1 may be essential for intestinal fibroblast binding of T cells. Musso et al concluded that intestinal fibroblasts may have a crucial regulatory role in mucosal immunity and that they may be potential targets for therapeutic intervention in intestinal inflammation.54 Our data proved evidence that interaction of CD40 with CD40L displaying T cells may induce ICAM-1 expression which then increases T cell adhesiveness and further improves the ability of fibroblasts to interact with T cells. Other chemokines relevant for the pathophysiology of IBD may be induced by CD40 ligation, as suggested by studies on fibroblasts from other origins.21,22,24,25,27,44,45 Expression of matrix metalloproteinases which are involved in tissue remodelling was also shown to be induced by CD40 ligation in vascular smooth muscle cells and in endothelial cells.
Induction and activation of CD40 on PCLF may therefore play a role during mucosal inflammation. As PCLF have an important role during formation of fistulae and especially strictures, further studies have to show whether the pathway described here is of pathophysiological relevance under those conditions. Particularly in patients with stricturing disease, inhibition of costimulated PCLF could yield therapeutic benefits.