Article Text
Abstract
Functional gastrointestinal disorders (FGIDs) are characterised by visceral pain or discomfort with an unknown cause. There is increasing evidence for abnormal processing of sensory input in FGIDs. Modulation of sensory input occurs at all levels of the nervous system, with a dynamic balance between facilitation and inhibition and close integration with the body's wider homoeostatic control. Cognitive, emotional, autonomic and spinal reflex pathways effectively orchestrate supraspinal and spinal pain modulation, as demonstrated in neurophysiological and brain imaging studies. Endogenous pain modulation has been studied in visceral pain conditions and abnormal regulation has been shown in irritable bowel syndrome (IBS) and functional dyspepsia, as well as other chronic pain syndromes. A majority of patients with IBS have diminished pain inhibition or even pain facilitation compared with healthy controls. Brain imaging during specific activation of endogenous pain modulation demonstrates a fairly consistent functional hub of mainly frontal, limbic and brainstem modulatory regions in healthy humans. Patients with IBS have a different pattern of activation and a correlation between the imaging and sensory changes. Because the modulatory balance of inhibition and facilitation appears to be distributed within the same functional network, future imaging studies of modulation mechanisms should include conditions allowing quantification of inhibitory and facilitatory components. An altered modulatory balance may well be a unifying pathophysiological mechanism in FGID as it can be driven by both top-down (ie, CNS pathology) and bottom-up (ie, peripheral immune activation) influences, but further validation in diverse FGID groups over time is required. Therapeutic manipulation of the modulatory system is possible by both pharmacological and non-pharmacological means.
- Functional bowel disorder
- acid secretion
- nerve—gut interactions
- visceral nociception
- neurogastroenterology
Statistics from Altmetric.com
- Functional bowel disorder
- acid secretion
- nerve—gut interactions
- visceral nociception
- neurogastroenterology
Introduction
Visceral pain is one of the most frequent reasons for medical consultation and is an integral part of the most common gastrointestinal syndromes—the functional gastrointestinal disorders (FGIDs) such as irritable bowel syndrome (IBS) and functional dyspepsia (FD). Recent evidence is transforming our concepts regarding the pathogenesis of visceral pain in FGIDs as a prominent number of affected individuals can now been shown to have either cognitive, behavioural or sensory dysfunction, or subclinical signs of immune activation, all with very similar clinical symptoms.1 Pain represents an integrated response of multiple body systems which affect homoeostatic control functions, such as autonomic nervous and immune regulation.2 Chronic pain is often accompanied by a lowered pain threshold (allodynia) and increased pain to a stimulus (hyperalgesia).3 This sensitisation may be driven by peripheral, spinal or central nervous system changes. Recent data suggest that an individual's pain response is dependent on the unique dynamic balance between pain inhibition and facilitation, providing at least a partial explanation for the widely differing pain responses.4 5 The balance between pain inhibition and facilitation is governed by a modulatory network of brain and brainstem regions closely linked to spinal pathways.6–8 Pain modulation has been shown to be abnormal in several chronic pain disorders (see box 1). The ability to favourably influence endogenous pain modulation (EPM), pharmacologically as well as cognitively, emphasises the clinical importance of these mechanisms. This paper discusses the role of the body's pain regulatory mechanisms in FGID. These mechanisms will collectively be referred to as EPM (see box 2).
Conditions in which endogenous pain modulation has been shown to be dysfunctional
Irritable bowel syndrome
Fibromyalgia
Functional dyspepsia
Chronic pancreatitis
Temporomandibular joint dysfunction
Increased postoperative pain/requirements for analgesia
Migraine
Tension-type headache
Osteoarthritis
Rheumatoid arthritis
Chronic trapezius myalgia
Neuropathic pain
Chronic widespread pain
Pain catastrophising
What is endogenous pain modulation and what is its relevance in gastroenterology?
All painful input is modulated at the levels of the peripheral nerve, the spinal and supraspinal (brainstem and brain) nervous system. These levels are intricately connected and modulate other homoeostatic mechanisms as well as pain.
Modulation is a dynamic balance between inhibition and facilitation, which is influenced by bottom-up (ie, spinal and peripheral) as well as top-down (ie, central nervous system) factors. This balance is highly individual and appears to correlate with different clinical measures of pain sensitivity.
Pain modulation is abnormal in irritable bowel syndrome as well as in other chronic pain conditions, with a decrease in the inhibition seen in health or a facilitation. This may be driven by peripheral inflammation as well as by psychological changes.
Endogenous pain modulation is strongly influenced by cognitive, analgesic and anti-inflammatory measures and may explain some of their therapeutic effectiveness.
Background
Physiology of EPM
Endogenous modulation of pain can be defined as the body's adaptation of incoming nociceptive information to momentary as well as long-term circumstances and needs. This definition closely parallels the definition of homoeostasis proposed by William Cannon in the early 1900s: the property of a living system to regulate its internal environment to maintain a stable constant condition.9 Pain modulation is a dynamic process balancing inhibition and facilitation and is intricately connected to other homoeostatic systems (figure 1).2 3 6 7 19
Factors known to affect the balance between pain inhibition and facilitation.10–18
Modulation can occur at all levels of the sensory nervous system—that is, the peripheral, spinal and supraspinal levels—as well as via the autonomic nervous system and is dependent on the context of the injury as well as endogenous factors such as the psychological and genetic background (figure 1). This review will concentrate on the central so-called descending modulation of pain, but a brief summary of modulation at the more peripheral levels is included for better understanding of the integration between all levels of pain processing (figure 2).
Interaction between levels of pain modulation. Inhibition and facilitation are determined interactively at every level of pain processing.
Peripheral pain modulation
Injury or inflammation can lead to persistent peripheral input and sensitisation. In IBS there is mounting evidence of sensitisation and of chronic low-grade intestinal immune activation probably associated with increased intestinal permeability.20 21 Upon tissue insult, pro-algesic and pro-inflammatory mediators are released by immune, neuronal, endothelial and epithelial cells. This algesic process is balanced by endogenous analgesic mechanisms including the release of analgesic and anti-inflammatory cytokines and endocannabinoids.22 Immunocyte- and leucocyte-derived opioid peptides released in the immediate vicinity of sensory neurons and acting on mu- (MOR), delta- (DOR) and kappa- (KOR) opioid receptors have a distinct local analgesic and anti-inflammatory effect.22–24 The analgesic effect is dependent on the presence of inflammatory cells and hence inflammation.
Spinal pain modulation
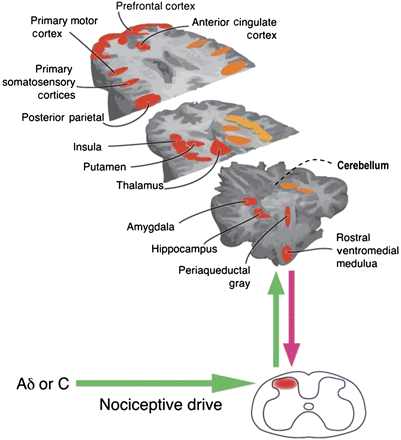
Extensive adaptation of peripheral afferent sensory input occurs at the spinal level, with a dynamic balance of inhibition and facilitation conferred by local interneurons and by multiple pathways originating supraspinally.6 25–29 Pro-inflammatory cytokines, chemokines, biogenic amines, bradykinins, prostaglandins, substance P, calcitonin G-related peptide and several neurotrophins are among the facilitatory mediators. Endo-opioids, anti-inflammatory cytokines and endocannabinoids tip the balance in favour of inhibition. Depending on the subtype of receptor activated, serotonin, dopamine and norepinephrine can have either excitatory or inhibitory function.6 30 Feedforward and feedback control of input is effected via spinal-supraspinal-spinal loops. Nociceptive input is conveyed from the dorsal horn to subcortical nuclei and then onwards to a hub of brain processing regions (figure 3).3 19
Brain areas consistently shown in imaging studies to be active in the processing and modulation of pain. Adapted from Tracey and Mantyh.31
Supraspinal (descending) modulation of pain
The function of the supraspinal pain control pathways is to either amplify or to subordinate noxious or potentially noxious stimuli in coordination with the individual's homoeostatic needs.2 Control of the brain over spinal input is both inhibitory and facilitatory and, importantly, the same brain centres can exert both inhibitory and facilitatory influences.3 7 19 The majority of research on descending pain modulation has concentrated on inhibition, which has led to an ambiguous interpretation of brain imaging data in visceral pain. Regulation of the dynamic balance between inhibition and facilitation occurs within the brainstem rostroventral medulla (RVM) - peri-aqueductal grey (PAG) axis and in connected brainstem and higher cortical centres.19 32–35 The brainstem modulation may not be restricted to pain but appears to extend to a wide range of homoeostatic mechanisms.2 The balance of modulation depends on the type of activated afferent nerve fibres, the duration, the type and the background state or context of the injury.30 Chronic and intense noxious stimulation involving C-nerve fibres more commonly leads to sensitisation and activates modulatory mechanisms more extensively than short-lasting input.19 36 This distinction may explain some of the divergent results in sensory studies of IBS and other chronic pain states.
Supraspinal modulation of pain is triggered by cognitive, emotional and autonomic influences via a hub of cortical and limbic regions bidirectionally connected to the modulatory brainstem nuclei, as well as efferent motor and autonomic centres (figure 3).4 6 8 10 11 37–43 The considerable functional overlap of so-called cognitive and limbic areas in recent connectivity functional MRI (fMRI) studies questions a clear identification of regions within the dynamic coalitions of networks as purely affective or cognitive.11 Cortical processes prominent in FGIDs—such as expectation, vigilance, memory and distraction—exert profound descending modulation via brainstem-spinal pathways including the so-called diffuse noxious inhibitory controls (DNIC).12 44 45 Isolated perceptional modulation of pain at a cortical level or by brainstem-spinal loops is unlikely.41–43 Descending control over spinal dorsal horn neuronal activity is through noradrenergic, opioidergic, serotoninergic, dopaminergic and cannabinoid pathways which themselves can be either inhibitory or facilitatory depending on the receptor subtype activation (figure 4).6 13 30 37 46–58
Descending pain modulation pathways identifying the main transmitter systems. Adapted from Benarroch.6
Modulation by the autonomic nervous system
Sensory processing from the viscera differs from the superficial organs in the extensive primary involvement of the autonomic nervous system (see recent reviews59–61). Parasympathetic vagal afferents contain large numbers of sensory fibres relaying information to brainstem regions and then onwards to forebrain centres.62 63 The overlap of these areas with those responsible for bodily homoeostasis and pain-related responses, such as illness behaviour, autonomic, emotional, motor and immune reactions, emphasises extensive visceral input convergence and integration.62 64 Vagal stimulation may attenuate pain both centrally and peripherally and has an anti-inflammatory effect.65 The sympathetic nervous system appears to exert little influence on pain in physiological conditions, but possibly plays a role in neuropathic pain states.48 An important efferent function of the sympathetic nervous system is the control of immune activation via the locus coeruleus, influencing inflammatory activity and the immune responses central to most forms of chronic pain.
Endogenous pain modulation (EPM) in FGIDs
The dynamic balance between pro- and anti-nociception and the integration with other bodily control mechanisms is clearly established in health. Changes in this equilibrium may well play an important role in chronic visceral pain syndromes, with a shift towards sensitisation and pain facilitation. In the following sections, evidence of changes in pain modulation in IBS as well as early data for FD is summarised. FGIDs are associated with several factors which potentially shift the balance of pain modulation such as stress, anxiety, chronic inflammation and hypervigilance as well as other pain comorbidities.1 66–69 Because of the extensive integration of the modulatory pathways, both bottom-up (eg, chronic mucosal immune activation) as well as top-down (eg, cognitive-limbic dysfunction) influences could drive the disequilibrium.
Sensory testing shows abnormal EPM in FGIDs
Evidence for altered endogenous sensory modulation in FGIDs exists at several levels. Quantitative sensory testing has confirmed visceral and—depending on the stimulation procedure—somatic hypersensitivity in a majority of patients with IBS.70–75 This suggests sensitisation, but is not proof of abnormal modulation. Several test procedures have been developed specifically to assess EPM (see box 3). The best characterised model is termed DNIC, heterotopic stimulation or heterotopic noxious conditioning stimulation, where a primary painful stimulation is applied alone and then simultaneously with a second distant conditioning stimulation which normally reduces the pain intensity of the primary stimulation (figure 5). DNIC mechanisms have been extensively validated both in health and in disease in animals and in humans with a flexion reflex (RIII reflex) or the closely correlated changes in pain ratings.76–84 They are classically postulated to engage spino-bulbo-spinal pain modulatory loops, but recent brain imaging studies have shown considerable involvement also of supraspinal and cortical regions.44 45 85 It should be pointed out that heterotopic stimulation does not separately identify the magnitude of inhibition and facilitation, but quantifies the summation of both effects. A further stepwise water immersion paradigm has also been developed.86 Using these techniques, either a decreased pain inhibition or a facilitation compared to controls has been demonstrated in a majority of patients with IBS from a variety of ethnicities (figure 6), and there was a good correlation between the magnitudes of abnormal pain modulation and of changes in pain sensitivity.44 45 75 87–90 The presence of both abnormal visceral and somatic pain modulation as well as sensitisation in IBS indicates a generalised rather than a specifically visceral sensory disorder. This pain modulation is not sufficiently explained by distraction or attentional effects, although emotional and cognitive effects feed into the same general modulatory network.44 91 92 In healthy controls, heterotopic stimulation generally achieves a pain inhibition of 15–35%, with some racial differences between Asians, Black Africans and Caucasians.76 90 93 Over the past few years work by our group has demonstrated abnormal modulation by heterotopic stimulation in 70–85% of patients with IBS, with facilitation rather than inhibition occurring in approximately 50% and weaker than normal inhibition in the majority of the remaining patients.44 45 75 More recently, abnormal EPM has also been demonstrated in patients with FD using a novel and reproducible heterotopic stimulation model with capsaicin-induced gastric pain and simultaneous thermal foot pain.94 In healthy controls the gastric pain was reduced by a highly significant 65% by heterotopic foot stimulation compared with an insignificant reduction in patients with FD.94 Furthermore, the magnitude of abnormal modulation in patients with FD correlated with their clinical symptom intensity.94 The data in FD needs confirmation in larger trials and in subsets of patients with FD. These simple psychophysical techniques demonstrate abnormal modulation in most but not all patients with IBS and FD, and further validation in large diverse groups of patients with FGIDs in longitudinal studies is advised. Integration of the assessment of EPM with studies of other potential disease mechanisms such as immune activation, autonomic dysregulation or hypervigilance is likely to lead to a more complete understanding of the underlying and heterogenous-appearing pathologies in FGID. Abnormal pain modulation has also been shown with these tests in fibromyalgia, which often overlaps with FGID, and in other chronic pain syndromes.86 95 96
Methods of assessing endogenous pain modulation
Sensory testing using heterotopic stimulation—that is, two simultaneous and distant painful stimulations (eg, rectal distension and thermal hand stimulation).
Stepwise limb immersion and withdrawal from thermal water stimulus.
Manipulation of pain processing by cognitive or emotional input—for example, by variation of expectation (placebo/nocebo), suggestion, distraction, stress or anxiety.
Brain imaging with fMRI or PET during modulation of pain.
Brain and spinal electrical activity changes during modulation of pain—for example, cerebral evoked potentials.
Model for testing endogenous pain modulation using heterotopic stimulation. Heterotopic stimulation denotes modulation of a primary pain stimulus by a second distant (heterotopically applied) painful stimulus. The change in primary pain stimulus intensity is the outcome measure. The graph shows typical results for pain intensity of primary pain alone (red bar), pain intensity of primary pain together with heterotopic stimulus (yellow bar) and the pain intensity decrease due to the heterotopic stimulus (green bar).
Endogenous modulation of (A) visceral pain (rectal pain) and (B) somatic pain (hand pain) using heterotopic foot stimulation in healthy controls (n=69) and patients with irritable bowel syndrome (n=84). Decreased pain inhibition or pain facilitation was seen in patients with irritable bowel syndrome compared with the consistent inhibition in controls. (C) Pain modulation differs significantly between individuals of different ethnicities (rectal pain, n=69). Means and 95% CIs are shown. Reproduced with permission from Wilder-Smith and Robert-Yap.75
Abnormal EPM in FGID by brain imaging
fMRI and positron emission tomography studies have been pivotal in elucidating the brain areas and networks activated during pain, discomfort and cognition-related tasks in health and FGID. However, the experimental context and study design require a critical interpretation regarding applicability to clinical situations. An in-depth review of these imaging studies is beyond the scope of this specialised paper and the reader is referred to excellent recent publications.97–102 Several fMRI and positron emission tomography studies with cognitive and sensory manipulation have shown differences in activation between patients with IBS or FD and healthy subjects in brain regions associated with EPM.103–105 In brain imaging studies, function is frequently ascribed to different regions and changes in activation are taken to imply parallel changes in function, with the accompanying risk of reverse inferencing.102 Aue et al, in a useful paper on this topic, suggest that investigators do not assume any invariant relationships but instead expect to have to marshal empirical evidence to support fMRI claims.106 In this context, a minority of imaging investigations were concerned specifically with modulation of experimental pain, and no formal imaging studies of clinical IBS or FD pain exist to the best of our knowledge. Consequently, we will first summarise the indirect evidence regarding pain modulation in FGID and then examine the studies with specific manipulation of pain modulation during imaging.
In studies of rectal distension, pain or discomfort, patients with IBS have fairly variable differences in activated brain regions compared with controls, generally comprising divisions of the prefrontal cortex, anterior cingulate cortex (ACC), mid-cingulate cortex (MCC), insula, amydala, hypothalamus and brainstem nuclei (figure 7).44 45 107–110 Many of these areas are implicated in pain processing as well as in cognitive and emotional processing. A clear distinction between different psychological modulatory regions (eg, for emotion and cognition) is problematic in healthy controls, but even more so in patients with FGIDs who have additional psychological dysfunction.11 107 111 112 Activation differences in these regions have been variously interpreted as abnormal function either in the pain facilitatory or the inhibitory pathways. While the described areas are part of the recognised modulatory network, current mechanistic studies do not delineate distinct facilitatory and inhibitory pathways but indicate that the balance of modulation is regulated largely within the same circuits.3 Some of the seemingly discrepant fMRI results may be explained by paradigm-related differences in balance or gain within the same network rather than different circuitry. The modulatory balance is likely to be set by cognitive factors very relevant in IBS, such as expectation, anxiety, learning processes and association.10 12 107 108 It should further be cautioned that the functioning of pain modulatory mechanisms at below-pain (ie, discomfort) intensity is presently unclear.
Brain and brainstem areas showing most consistent activation differences between patients with irritable bowel syndrome and healthy controls during imaging studies of rectal pain or discomfort. ACC, anterior (posterior-anterior) cingulate cortex; AMY, amygdala; HYP, hypothalamus; INS, insula; MCC, middle cingulate cortex; PAG, ????; PFC, prefrontal cortices.
Hypotheses for CNS as well as peripheral aetiologies exist in FGID, and there is supportive evidence for both in fMRI studies. Top-down modulatory dysfunction in IBS, corresponding to attentional and cognitive changes such as hypervigilance and increased symptom-directed anxiety, is visible in the abnormal prefrontal cortex activation patterns.113 The diminished prefrontal activation often demonstrated in IBS is also associated with negative emotions and increased pain.12 114 The success of hypnosis in IBS may reflect a modulatory effect with increased prefrontal activation feeding into the downstream modulatory areas, resulting in distancing from the emotional impact of pain and in pain reduction.115–117 On the other hand, there is also evidence in imaging studies of increased intestinal (peripheral) sensory input due to sensitisation or subcortical facilitation in IBS. Thus, with identical rectal stimulus intensity, brain activation in the early pain processing areas was greater in IBS than in controls whereas, in subjective rating-matched stimulation, there were only minor activation differences in these areas between subject groups.45 118 Several recent interesting studies have examined the linkage between the main pain processing centres in IBS using connectivity analysis.108 119 120 However, owing to the absence of adequate control groups, they can only provide indirect evidence of any disease-related changes in modulation and will not be considered here.
Brain activation differences during a sensory stimulus between patients with IBS and controls are frequently ascribed to abnormal endogenous sensory inhibition. While associations are possible in these studies with generally smaller numbers of subjects, certain caveats need to be mentioned as the associations between imaging and function are unlikely to be linear for several reasons: the complex relationship between neuronal activation, perception and BOLD fMRI signal change; the involvement of the same brain centres in both inhibitory and facilitatory actions; and a shifting modulatory balance with chronic input. Furthermore, many of the classic modulatory brain centres are also extensively involved in regulation of non-nociceptive functions. To address these issues, several studies have included paradigms actively engaging quantifiable pain modulation during imaging. These paradigms include attentional and emotional modulation, placebo and nocebo procedures and the above-described heterotopic stimulation. All of these procedures significantly and quantifiably alter pain intensity but, nonetheless, they bear the limitations inherent in the application of acute experimental procedures to a chronic disease setting. We will consider the relevant studies relating to IBS below.
Brain imaging during activation of EPM by heterotopic stimulation in IBS
Heterotopic stimulation has demonstrated abnormal pain modulation in IBS and, consistent with this, concurrent brain imaging has shown aberrant brain activation in areas associated with the cognitive and emotional modulation of pain (figures 8 and 9).44 45 75 114 121–123 The processing seen in the classic modulatory regions during effective pain inhibition in controls contrasts with the increased activations in the so-called ‘fear and threat’ network in IBS during pain facilitation or decreased inhibition. Moreover, the diminished endogenous pain inhibition in patients with IBS correlated with the activation changes in the key pain processing areas and also with the presence of somatic and visceral hyperalgesia.44 45 Analysis of functional brain connections during pain modulation induced by heterotopic stimulation showed top-down control of cortical brain areas over key brainstem modulatory areas in healthy controls but a reversed bottom-up direction of control in patients with IBS.121 The predominantly quantitative rather than qualitative activation differences in IBS imaging studies may well be due to a shifted gain within the modulatory balance in these central control areas, as has been demonstrated in various forms of chronic pain in animals by Vanegas and Schaible.19 In summary, current pain modulation brain imaging studies support the presence of abnormal EPM in IBS. We are not aware of any brain imaging studies during explicit modulation of pain in FD.
Brain and brainstem areas showing most consistent activations in patients with irritable bowel syndrome (red) and healthy controls (blue) in imaging studies during activation of pain modulation using heterotopic stimulation. ACC, anterior cingulate cortex; AMY, amygdala; DBS, dorsal brainstem (periaqueductal grey); HIP, hippocampus; INS, anterior insula; PAR, parietal cortex; PCC, posterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; mPFC, dorsolateral prefrontal cortices; RVM, rostroventral medulla; S1, primary somatosensory cortex; THAL, thalamus.
Brain functional MRI showing regions activated during endogenous pain modulation by heterotopic stimulation (painful rectal distension with foot cold pain) in healthy controls (left column) and patients with irritable bowel syndrome (right column). Reproduced with permission from Song et al.44
Brain imaging during psychological modulation of pain and IBS
The individual experience of pain is powerfully influenced by cognitive and emotional mechanisms such as the expectation and desire of pain relief (placebo), the expectation of pain (nocebo), memory, reward and mood.124 125 Expectation may explain up to 50% of the variation in pain ratings.125 Placebo and nocebo effects appear to represent a continuous spectrum of responses rather than qualitatively different mechanisms.126 This balance between hypo- and hyperalgesia, or inhibition and facilitation, is predominantly regulated by endogenous opioid and cholecystokinin systems.127 Cholecystokinin is implicated in hyperalgesia mediated in the PAG, counterbalancing opioid-induced analgesia, and also in the pathogenesis of IBS.128–132
Brain imaging studies with placebo in healthy individuals have shown a correlation between the degree of expected pain relief and brain activation in main modulatory (especially opioidergic) areas.14 50 52 101 133–136 However, dopaminergic reward pathways in the basal ganglia also account for approximately 25% of the variance of placebo responses.54 Placebo analgesia manipulates activity in cortical and subcortical regions key to the cognitive and emotional modulation of pain. Importantly, the anticipation of pain and of nocebo-induced hyperalgesia activated very similar brain regions.15 16 136–140 The overlap in brain areas activated during cognitively-induced pain inhibition and pain enhancement again suggests a modulation executed within an overlapping functional network.
Comparatively few brain imaging data have been published examining cognitive and emotional effects on pain in FGID. However, these modulatory mechanisms are of particular relevance in FGID, where memory of abuse, somatisation, negative mood, symptom-related anxiety, selective attention and hypervigilance are implicated in pathogenesis and where expectation and placebo responses are considered elevated.107 109 141 In patients with IBS the history of abuse was associated with activation differences compared with controls in distinct subregions of the cingulate cortex during rectal distension.142 In a separate study, increased anxiety during expectation of a rectal distension in patients with IBS correlated with an absence of deactivation in arousal network regions, especially the dorsal brainstem.110 Therefore, although associations between psychological processes, gastrointestinal sensory function and symptoms have been shown in patients with FGID during functional brain imaging, most studies have been performed in uncontrolled settings in IBS.107 118 143–146 Rectal placebo with the suggestion of enhanced analgesia in IBS reduced both rectal distension pain ratings and brain activations in divisions of the insula and ACC compared with rectal distension alone.143 Functional connectivity analysis yielded differences in the direction of influence within modulatory areas with placebo.120 Further controlled studies are needed to better characterise the psychological modulation of pain in FGID and visceral pain in general.
Therapeutic implications of EPM in FGID
The descending pain modulatory pathways can be manipulated with a wide range of medications including opioidergic, serotoninergic, noradrenergic, dopaminergic and non-steroidal anti-inflammatory drugs.40 78 147 Given the close integration of cognitive, emotional and modulatory processes, it would be expected that behavioural and cognitive treatments exert their principal effects via the modulatory pathways. Indeed, imaging and psychophysical data provide corresponding supportive evidence for placebo and nocebo studies, and data emerging from trials of hypnosis, cognitive behavioural therapy, mindfulness and dynamic psychotherapy indicate that their potential effectiveness in the treatment of FGID may be via the modulatory networks.125 128 148–151 There are therefore considerable pharmacological and non-pharmacological therapeutic possibilities of manipulating EPM that deserve further exploration with high-quality studies.
Conclusions and future directions
There is increasing evidence for abnormal EPM in IBS and FGID, with a shift in balance from inhibition towards facilitation. EPM may be part of the body's integrated and dynamic homoeostatic response and probably explains some of the related changes in other regulatory systems in FGID such as autonomic function, immune, cognitive and gastrointestinal motility changes. Both top-down (ie, CNS dysfunction) and bottom-up (ie, spinal and peripheral immune activation) could reset EPM (figure 10). Undoubtedly, the very loose phenotypical definition of FGID encompasses different subgroups of pathologies, however disordered function of a central regulator of homoeostatic functions would go far in explaining some of the divergent manifestations. As Sherrington suggested in 1900: ‘Pain is a curiously imperative occurrence that co-opts descending bulbospinal neurons to make necessary adjustments to sensory, autonomic and motor functions’.152
Factors potentially driving changes in endogenous pain modulation in visceral pain syndromes. Shifted modulatory balance may act as a central mechanism in chronic pain syndromes and may predict an individual's pain sensitivity.
Brain imaging is increasingly defining the predominant structures involved in the modulation of pain. The pathways and centres governing the balance between pain inhibition and facilitation largely overlap. Future imaging studies should therefore include conditions allowing quantification of inhibitory and facilitatory components. Comparisons between healthy controls and patients with FGID incorporating the assessment of peripheral, spinal and supraspinal modulation during placebo and nocebo manipulation are necessary to further define the aberrant pain modulation in FGID.
References
Footnotes
Competing interests None.
Provenance and peer review Commissioned; externally peer reviewed.