Article Text
Abstract
The gut microbiota has recently evolved as a new important player in the pathophysiology of many intestinal and extraintestinal diseases. The liver is the organ which is in closest contact with the intestinal tract, and is exposed to a substantial amount of bacterial components and metabolites. Various liver disorders such as alcoholic liver disease, non-alcoholic liver disease and primary sclerosing cholangitis have been associated with an altered microbiome. This dysbiosis may influence the degree of hepatic steatosis, inflammation and fibrosis through multiple interactions with the host's immune system and other cell types. Whereas few results from clinical metagenomic studies in liver disease are available, evidence is accumulating that in liver cirrhosis an oral microbiome is overrepresented in the lower intestinal tract, potentially contributing to disease process and severity. A major role for the gut microbiota in liver disorders is also supported by the accumulating evidence that several complications of severe liver disease such as hepatic encephalopathy are efficiently treated by various prebiotics, probiotics and antibiotics. A better understanding of the gut microbiota and its components in liver diseases might provide a more complete picture of these complex disorders and also form the basis for novel therapies.
- ALCOHOLIC LIVER DISEASE
- HEPATIC ENCEPHALOPATHY
- LIVER CIRRHOSIS
- PRIMARY SCLEROSING CHOLANGITIS
Statistics from Altmetric.com
Introduction
Non-communicable diseases, also known as chronic diseases are still growing around the globe. According to WHO, the four main non-communicable diseases are cardiovascular diseases, diabetes, cancer and chronic respiratory diseases. Strikingly, although our genetic background has not changed over the last century, the last three decades have been clearly associated with the globalisation of such diseases. The key triggering factors for many diseases associated with lifestyle include our changes in eating habits, exposure to specific xenobiotics or alcohol and tobacco consumption. Today, the role of the inhabitant of our gut, the so-called gut microbiota is also becoming recognised as a major environmental factor.
We knew from several decades that as human, we are composed of trillion cells, whereas the number of bacteria present in our gut and living in symbiotic harmony with our body is estimated to also reach such a comparable vast number of cells (eg, human body: around 3×1013 eukaryotic cells; the microbiota around 4×1013 colonising microbes).1
In utero, mammals are already exposed to microbiota or derived compounds, and this exposure expands rapidly after birth.2 ,3 Therefore, it is suggested that in adults the gut microbiota composition and its activity may reflect in part the history of exposure to bacteria and environmental factors during early life. The divergences observed in composition of human gut microbiota are likely the result of multiple factors. For example, individual factors such as the genetic background, the age, the development of the immune system, the geographical location or any dietary modifications of short or longer durations may influence the gut microbiota composition.4–10
However, better understanding the correlations and eventually the potential causal link existing between the intestinal microbial community and the onset of metabolic disorders requires a comprehensive knowledge of the gut microbiota. Over decades, several methodological approaches have been used and are divided into two major categories: culture-dependent and culture-independent approaches. Culture-based techniques were the major methods used and are still used to analyse the microbiota composition. However, so far about only 30% of the gut microbes have been cultured. This does not mean that 70% of the gut microbiota are unculturable but simply suggests that it is very difficult to identify and to develop the optimal growth conditions for these organisms. This is likely due to the fact that the microbiota is a complex ecosystem, which is not easy to decode. Therefore, using culture-based methods alone is not suitable to provide a complete view of the resident microbes.
Although each technique has its own advantage or limitation, culture-independent methods have been useful to better illustrate the complexity of the microbiota composition. Thus, one may argue also that the choice for a specific approach depends on the key scientific questions that should be addressed. Among these methods, quantitative PCR (qPCR) and fluorescence in situ hybridisation have the advantage to be both highly sensitive, and eventually suitable to quantify specific bacterial groups. Conversely, using fingerprinting techniques allow a rapid comparison of profiles to identify the most abundant phylotypes (eg, within the same individual or between pathological situations), but, these methods require an a posteriori identification of specific bands by sequencing or hybridisation to identify known or unknown bacteria. More recently, phylogenetic microarrays have been developed and therefore used as a high-throughput technique.11 This method has contributed to progressively increase the knowledge of microbiota composition and is considered as a fast and semiquantitative method.12 However, the major caveat is that the detection of any species depends on the presence of known reference sequences on the array. As a consequence, this technique is not suitable for discovering novel taxa.
Over the last few years, next-generation sequencing methods have been developed and have dramatically increased the number of novel data and generated gigabases of sequences in one single run. The major advantage of these techniques is to allow to determine the relative abundance of both known and unknown bacteria.13 In most of the studies available, both culture-dependent and culture-independent methods have been used. Thus, thanks to the recent development of such analytical tools, the scientific community is progressively starting to highlight at least in part the composition of the human intestinal microbiota.14 For example, different catalogues of up to 10 million non-redundant genes have been published15 ,16 and novel taxa have recently been identified or associated with diseases progression.17–20
In general, it is considered that a healthy microbiota-host symbiosis is resulting from a stable GI tract environment, rich in nutrients and providing mutualistic interests. The microbiota will ensure to provide essential nutrients (eg, production of vitamins, increase bioavailability of essential nutrient) by assisting digestion of non-digestible compounds (eg, dietary fibres), thereby allowing the growth of a metabolically active population of bacteria. It is commonly accepted that any perturbations of the relationship existing between host and microbes may be associated with the pathogenesis of several major diseases, thereby highlighting the role of microbes outside the intestinal tract. However, while important progress has been achieved thanks to metagenomics analyses, and association between specific taxa and diseases, a vast number of key questions remain unanswered, including the demonstration of a causal link in the onset of non-communicable diseases such as obesity, diabetes and cardiometabolic diseases. Nevertheless, it is now widely accepted that the gut microbiota composition and its metabolic capacity contribute to regulate host metabolism. We will herein limit our discussion on a key organ which is at the interface of many interactions between the gut and host metabolism, that is, the liver.
Non-alcoholic fatty liver disease, metabolic inflammation and microbiota
The liver is structurally and functionally heterogeneous and contains a large number of different cell types, such as hepatocytes, immune cells (innate and adaptive immune system) including Kupffer cells, lymphocytes, but also stellate cells and progenitors.
The liver is at the crossroad between the portal blood flow coming from the intestinal circulation and peripheral organs. This anatomical position offers to the liver specific interactions with many compounds coming from the digestion of nutrients transiting throughout the gut and also with other microbiota-derived signals. In physiological situations, nutrients and bacterial compounds are delivered to the liver via the portal circulation and contribute to the host homeostasis. Thus, the major border between the intestinal lumen (ie, microbes, digested food, xenobiotic), the portal blood and the liver is the gut barrier. This barrier comprises the intestinal mucosa of the host and also several other highly complex and dynamic cells and structures that are at the interface between the microbiota, the liver and other organs.
Although the gut lining acts as an active barrier against the translocation of bacteria and/or microbial-derived products, a small quantity of these compounds may enter the portal venous blood. Thus, a fine-tuned sensitive balance takes place to limit excessive responses between an expected protective immune response against exogenous insults and immune tolerance.
The pathogenesis of different liver diseases has been associated with changes in the gut microbiota composition. Among these, non-alcoholic fatty liver disease (NAFLD) is a common clinical syndrome, induced by factors other than alcohol consumption or any other well-established liver injury. NAFLD is pathologically characterised by a diffuse accumulation of fat in the liver cells (steatosis) and is also a key factor involved in the development of insulin resistance, type 2 diabetes and cardiovascular risk. Moreover, a certain proportion of patients with NAFLD will evolve into non-alcoholic steatohepatitis (NASH) and eventually cirrhosis and hepatocellular carcinoma.21
Gut microbes and hepatic steatosis: mechanisms
Studies have revealed that the alteration of gut microbiota may play an important role in the development and progression of NAFLD. For example, more than 20 years ago, seminal papers have shown that changing the microbiota composition by using prebiotics such as inulin-type fructans reduces hepatic steatosis and de novo lipogenesis.22–25 These studies were the first showing a decrease in plasma triglycerides and very low-density lipoprotein production in prebiotic-fed rats. The triglycerides-lowering action of prebiotics is mediated by the inhibition of all lipogenic enzymes, namely acetyl-coenzyme A carboxylase (ACC), fatty acid synthase (FAS), malic enzyme, ATP citrate lyase and glucose-6-phosphate dehydrogenase (figure 1).26 In 2003, Letexier et al27 showed that changing the microbiota by using prebiotic decreased hepatic lipogenesis and plasma triglycerides, thereby showing one of the first metabolic links between microbiota and liver in humans. The fermentation of prebiotics by gut microbes increases the abundance of short chain fatty acids in the caecum and also in the portal vein blood, where the concentration of both acetate and propionate is doubled.28–30 Data suggest that propionate may contribute to reduce hepatic lipogenesis, whereas acetate is a lipogenic substrate (figure 1).31 ,32
Prebiotic fermentation reduces hepatic steatosis. Gut microbiota is using prebiotic and fermentable carbohydrates as energy sources; hence, short chain fatty acids (SCFA) production such as propionate may cross the gut barrier and reach the liver through the portal vein blood. Propionate inhibits lipogenesis by acting on the transcription of several rate-limiting step enzymes involved in de novo lipogenesis. As a consequence, these fibres and their metabolites are putative tools to reduce steatosis and inflammation. ACC, acetyl-coenzyme A carboxylase; FAS, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase.
In 2005, Daubioul et al33 showed in a pilot study with seven patients with NASH (confirmed by biopsy) that after 8 weeks, prebiotic feeding decreased significantly hepatic inflammatory markers. More recently, 66 patients with NASH were randomly divided into two groups. The first group receiving a symbiotic (ie, mix of Bifidobacterium longum and prebiotics (inulin-type fructans) combined with lifestyle modification, whereas the second group received a placebo with lifestyle modifications.34 After 24 weeks of treatment, the authors found that the modulation of the gut microbiota significantly reduced inflammatory markers (ie, tumour necrosis factor-α, C reactive protein) as well as steatosis and NASH activity index. Altogether, these data strongly suggest that the metabolic activity of microbes may contribute to the regulation of hepatic lipogenesis and eventually the development of steatosis. But, it is worth noting that a causal impact of specific bacteria or metabolites was not shown in these studies.
However, this causality between weight gain, fat mass and steatosis has been shown by Bäckhed et al,35 who discovered that mice raised in the absence of microorganisms (axenic or germ free) exhibited a lower total body fat mass than mice harbouring a normal gut microbiota. They also found that to conventionalise germ-free mice with a gut microbiota from normal mice increases body fat content, insulin resistance and liver fat.35 The authors observed that colonising the gut with microbiota was accompanied by a twofold increase in hepatic triglyceride and a higher hepatic de novo lipogenesis measured by the expression of several key factors and enzymes such as hepatic carbohydrate responsive element binding protein, sterol responsive element binding protein 1, ACC and FAS.35 Among the mechanisms proposed, the authors found that conventionalised mice had an increased intestinal saccharides absorption and delivery of glucose to the liver that eventually participates in higher lipogenesis (figure 1).
Another piece of evidence supporting a role for the gut microbiota in the development of NAFLD has been demonstrated by Le Roy et al. They observed that upon a high-fat diet treatment, a certain proportion of mice develop obesity, insulin resistance and NAFLD. Strikingly, in such set of rodent experiments, the genetic background is exactly the same as well as the dietary environment; however, for different unknown reasons the microbiota may respond differently to diet. Despite a majority of the mice investigated do develop metabolic disorders (ie, higher glycaemia, systemic inflammation and steatosis), some other were resistant to the diet-induced metabolic disorders and were considered as ‘non-responders’ versus ‘responders’. Interestingly, the microbiota composition of ‘non-responders’ was different from ‘responders’. By using germ-free mice, they demonstrated that the susceptibility to develop NAFLD was transmissible by the gut microbiota transplantation. Thus, this set of experiments clearly shows that specific bacterial species are associated with the onset of NAFLD upon a dietary trigger such as high-fat feeding.36
Dysbiosis of the gut microbiota has been associated with NAFLD in mice and in humans.37 A recent review by Abdou et al38 discusses multiple studies performed in humans showing that dysbiosis is accompanied by an abnormal shift of the gut microbiota as compared with healthy individuals. However, there is still no clear consistency or specificity towards key gut microbes to explain predisposition to develop NAFLD.
Although this is not the scope of this review, evidence suggests that obesity and insulin resistance are the key risk factors for development of NAFLD. Numerous recent studies have shown that the gut microbiota composition and its activity are different during obesity and type 2 diabetes.5 ,39–42 Although, it is still too early to link these metabolic situations with the systematic presence or the absence of specific taxa, there is a consensus on the fact that during obesity and diabetes, the bacterial diversity is lower than in healthy subjects. Recently, Le Chatelier et al identified that the number of bacterial genes (ie, microbial gene richness) follows a bimodal distribution of microbial genes. This led the authors to propose a stratification of subjects on either harbouring ‘low gene count’ (LGC) or ‘high gene count’ (HGC) according to the number of genes present in their microbiome.40 Interestingly, specific taxa have been linked with both the metabolic situations and the gene counts. For example, the authors proposed that butyrate producers such as Anaerotruncus colihominis, Butyrivibrio crossotus, Faecalibacterium prausnitzii and Roseburia inulinivorans were less abundant in patients with LGC and may be associated with an obese phenotype. In addition, other specific beneficial microbes such as Bifidobacterium spp and Akkermansia muciniphila were also less abundant. Conversely, increased abundance of Ruminococcus gnavus, Ruminococcus torques, Campylobacter and Shigella was observed.40 A recent study has found an association between the abundance of Prevotella copri, Bacteroides vulgatus and insulin resistance in humans. They also described that P. copri aggravates insulin resistance and triggers glucose intolerance in rodents, thereby showing a putative causal link.43 Interestingly, Dao et al found in obese humans, the abundance of A. muciniphila was inversely related to visceral fat mass, fasting glycaemia and adipocyte size. They also observed that subjects categorised as HGC and higher A. muciniphila abundance exhibited improved insulin sensitivity markers and other cardiometabolic risk factors.44 It is worth noting that this bacterium has been shown to protect against diet-induced obesity, reinforce the gut barrier and reduce low-grade inflammation in rodents.18 Therefore, although the exact implication of the taxa mentioned earlier in the onset of metabolic disorders remains to be proven in humans, several studies are now consistently showing such kind of associations.
From the gut to the liver: impact of inflammation
Gut bacteria are able to interact with host cells via specific molecules called pattern recognition receptors (PRRs). These PRRs will recognise particular molecular patterns of bacteria and other microorganisms, namely the pathogen-associated molecular patterns (PAMPs). Among the different PRRs, toll-like receptors (TLRs) are the most studied. In 2007, Cani et al discovered that gut microbes were involved in the onset of insulin resistance, low-grade inflammation and diabetes, by activating TLR signalling pathways. They found that constituents of Gram-negative bacteria (ie, lipopolysaccharides (LPS)), which are circulating at a very low level in the blood, were able to trigger low-grade inflammation and altered glucose metabolism.45 This phenomenon was called metabolic endotoxemia (figure 2).
Disease progression model from normal liver to non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): contribution of the gut-liver axis. The gut lining is composed of several actors (eg, mucus layer, symbiotic gut microbes) contributing to maintain the gut barrier. Changes in dietary habits such as reducing the ingestion of fibres together with higher consumption of saturated fat, alcohol and specific xenobiotics may contribute to change the microbiota composition and to alter the gut barrier function. The subsequent leaky gut is associated with the translocation of bacteria or fragments of bacteria that trigger the accumulation of liver fat and inflammation. Both NAFLD and NASH are associated with specific changes at the level of the immune response, as well as endogenous production of bioactive lipids. This dysbiosis is also associated with the production of potential organic compounds reaching the liver and changing its metabolic activity. Altogether, several mechanisms are involved in the onset of NAFLD and NASH, most of them being characterised by a central role of the gut microbiota. HFD, high-fat diet; PAMP, pathogen-associated molecular pattern; VOC, volatile organic compound.
Besides the apparent inflammatory tone and insulin resistance, they found that metabolic endotoxemia also triggers liver fat accumulation.45 This effect was abolished in mice lacking the LPS receptor complex CD14/TLR4,45 ,46 thus showing a direct link between gut microbial compounds and the development of hepatic steatosis. Along this line, as described earlier in this review, changing the microbiota by using prebiotics reduces hepatic steatosis and both hepatic and systemic inflammation in both rodents and humans (figure 1).33 ,34 ,47
Inflammasomes are considered as key sensors for endogenous and exogenous PAMPs or damage-associated molecular patterns (DAMPs). They control cleavage of pro-inflammatory cytokines such as pro-interleukin (IL)-1β and pro-IL-18. In 2012, Henao-Mejia et al showed that genetic inflammasomes deficiency induced a change in the gut microbiota composition. They illustrated that the genetically induced dysbiosis led to abnormal accumulation of bacterial products in the portal circulation and consequently increased hepatic steatosis.48 The role of inflammasomes in the onset of NAFLD has been recently reviewed (figure 2).49
More recently, Duparc et al investigated the impact of hepatocyte-specific deletion of myeloid differentiation primary response gene 88 (MyD88), a central adaptor molecule for the majority of these TLRs (with the exception of TLR3 and certain TLR4 signals), and members of the inflammasomes (IL-1 receptor and IL-18 receptor). They found that in absence of MyD88 specifically in the hepatocyte, mice were predisposed to glucose intolerance, inflammation, liver fat accumulation and hepatic insulin resistance, and this was independent of body weight and adiposity.50 In addition, this deletion profoundly affected the gut microbiota composition, therefore, showing that host genetics also contribute to shape the microbiota and in turn host metabolism. Among the potential mechanisms, they found that hepatocyte MyD88 is involved in the control of the synthesis of bile acids and several bioactive lipids, all involved in the regulation of glucose, lipid metabolism and inflammation.50
Interestingly, Duparc et al found that in human obese subjects who developed NASH, different factors involved in the production of bioactive lipids synthesis (ie, different cytochromes P450) were decreased, they also linked several transcriptional factors with the switch from NAFLD to NASH. However, the microbiota of these patients was not studied.50
Together, this suggests the existence of a crosstalk between microbes and host liver cells that control metabolism. In parallel to PAMPs, DAMPs and bioactive lipids, recent data suggest that volatile organic compounds (VOCs) produced by the microbial metabolism may differentiate obese patients with NAFLD from healthy individuals (figure 2).51 It is well known that the absorption of VOCs such as ethanol and other toxic molecules from the gut may be deleterious for the liver. However, the recent discovery that more than 40 gut-derived VOCs are present in the portal vein of mice developing NAFLD strongly suggests that besides the gut microbiota composition the metabolic activity of bacteria may expose the liver to xenobiotic compounds (figure 2, box 1).
Microbiota and non-alcoholic fatty liver disease (NAFLD)
Gut microbiota contributes to the regulation of de novo hepatic lipogenesis
Specific nutrients such as fat and alcohol change microbiota composition in a harmful manner, whereas prebiotics counteract these effects
Both innate immune system and xenobiotic metabolism control liver lipid metabolism via mechanisms involving bacterial components and metabolites
Hepatic innate immunity controls liver bioactive lipids production and contribute to switch from NAFLD to non-alcoholic steatohepatitis.
Alcoholic liver disease and microbiota
The diverse clinical picture of alcoholic liver disease (ALD) might be caused by various confounders including genetics, immunity, dietary components and the gut microbiota.52 An altered microbiota or commonly phrased ‘dysbiosis’, an impaired intestinal barrier and endotoxemia are well-known features of advanced ALD.53 Mutlu et al54 studied the mucosa-associated colonic microbiome in alcoholics with and without ALD and in healthy subjects. A subgroup of patients exhibited a dysbiosis with lower median abundances of Bacteroidetes and higher ones of Proteobacteria. Interestingly, changes corrrelated with endotoxemia. Intestinal permeability and dysbiosis, however, might also play a role in other alcohol-related aspects such as alcohol-dependence severity.55 This study suggested that indeed a gut-brain axis might exist in alcoholism providing certain evidence that the gut microbiota regulates behavioural disorders such as alcohol dependence. Therefore, it has been increasingly recognised that the gut microbiota might be relevant in alcohol-related human disorders.
The microbiota might be of exceptional relevance in severe alcoholic hepatitis (AH).56 Llopis et al56 showed that disease severity of human AH can be transferred via faecal microbial transplantation (FMT) both to germ-free (GF) and conventionalised mice. First, the authors characterised the gut microbiota of various groups of patients with AH describing that AH-related dysbiosis was characterised by an increase in Bifidobacteria, Streptococci and Enterobacteria, whereas certain species such as Clostridium leptum or F. prausnitzii, both well-established anti-inflammatory strains, were decreased.17 These protective strains were also negatively correlated with certain clinical parameters such as bilirubin levels. In FMT experiments, where stool from patients with or without AH was used, the authors found that severity of liver disease in animals was dramatically aggravated after performing FMT from a patient with severe AH. In contrast, if for FMT a human donor without severe AH was used, disease phenotype was mild. Importantly, disease could also be transferred to conventional mice and a second subsequent FMT from an alcoholic patient without AH even improved liver disease after having received FMT from a donor with severe AH before. This important study delivers some key messages to this rapidly evolving and exciting field: (i) there is a clear microbiota signature in severe AH, (ii) disease can be transferred from man to mouse and (iii) thereby suggesting that certain ‘pathobionts’ might indeed exist. It seems now crucial to enforce further studies trying to identify members of the microbiota, which are drivers of this often fatal disease in order to define new targets for better therapies.
Whereas as discussed certain members of the microbiota might drive ALD (ie, pathobionts), other members might exert beneficial effects in the disease process and act protective. This fits with recently presented data where Chen et al57 showed that ALD is aggravated in germ-free mice and in these studies hepatic ethyl alcohol (EtOH) metabolism was enhanced and susceptibility to binge-like alcohol drinking increased. Another exciting research area deals with the key questions how an intact intestinal microbiota is regulated. Local intestinal immunity including defensins and other antimicrobial factors might constitute major players in the regulation of the microbiota.58 Chronic alcohol consumption decreases intestinal levels of antimicrobial-regenerating islet-derived (REG)3 lectins. Intestinal-specific deficiency in REG3B or REG3G increased numbers of intestinal bacteria and their translocation to mesenteric lymph nodes and the liver aggravating ethanol-induced steatohepatitis. In contrast, overexpression of REG3G in the intestinal epithelium protected animals from ethanol-induced liver injury (figure 2). This overexpression was directly associated with reduced bacterial colonisation in the intestinal mucosa and reduced bacterial translocation. This study highlights besides the potential contribution of the gut microbiota to disease pathogenesis the importance of intestinal immunity.
Diet might reflect another major confounder in ALD, with the potential to either improve or aggravate underlying disease. Indeed, an unsaturated fat diet (corn oil enriched) exacerbated ethanol-induced endotoxemia and worsened liver disease in contrast to a saturated diet enriched in medium chain triglycerides.59 An unsaturated diet was accompanied by additonal alterations in the gut microbiota with reduction in Bacteroidetes and an increase in Proteobacteria and Actinobacteria. This study highlights the importance of dietary factors in alcohol-related liver disease potentially by manipulating the gut microbiota. To conclude, the gut microbiota has evolved as major player in ALD. Alcohol disrupts the intestinal microbiome, alters the intestinal barrier and might affect various other intestinal functions such as mucosal immunity. Therapies such as various diets, prebiotics and probiotics might have the potential to influence and correct these disturbances and appear as potential future therapies for these diseases.60 In addition, manipulation of the gut microbiota by prebiotics/probiotics or FMT selectively targeting key pathobionts might reflect another promising treatment strategy. Treatment of ALD and AH has entered a new exciting area (box 2).
Microbiota and alcoholic liver disease
Human alcoholic liver disease is characterised by a profound dysbiosis
An impaired intestinal epithelial barrier is an early event in many experimental models of alcoholic liver injury even preceding intestinal dysbiosis/altered microbiome
Impaired GI barrier might result in rapid endotoxemia
An altered intestinal microbiome is observed in various experimental liver disease models and microbiome changes might be disease-specific
Preclinical and clinical studies suggest an important role for the gut microbiota in alcohol-related disorders
Primary sclerosing cholangitis and microbiota
Primary sclerosing cholangitis (PSC) is commonly observed in patients with IBD and recent studies have investigated the gut microbiome in this disorder.61 Here, 16S rRNA gene sequencing performed in 85 patients with PSC demonstrated that such patients independent of the presence of IBD had a reduced bacterial diversity compared with healthy controls. Interestingly, approximately 11 genera were reduced in PSC, whereas Veilonella genus showed an increase. In summary, this first PSC study observed a clear gut signature in PSC distinct from healthy controls and patients with UC without liver disease suggesting that the intestinal microbiota (IM) could reflect a relevant player in PSC. These findings are strengthened by another recently published study where authors also observed an increase in Veilonella species.62 Veilonella has been associated with other chronic inflammatory disorders including fibrotic conditions and liver cirrhosis where subjects have higher colonic Veilonella levels.63 Another smaller study investigated mucosa-associated microbiota in 20 patients with PSC, 19 with accompanying IBD.64 Investigators observed an increase in mucosal Barnesiellaceae at the family level and Blautia at the genus level. Germ-free, multidrug resistance 2 knockout mice (mdr2−/− mice) show exacerbated biochemical and histological features of PSC and increased cholangiocyte senescence further supporting the notion that gut microbiota might play a role in disease process.65 Interestingly, primary bile acids were similar, whereas secondary bile acids were absent in germ-free mdr2−/− mice. This study also implicates an important role for the gut microbiota and certain metabolites such as secondary bile acids in the protection against biliary injury. PSC and microbiome research is a rapidly emerging topic and one of the most challenging issues will remain to understand and identify differences in the microbiome of patients with PSC with and without IBD (box 3).
Microbiota and primary sclerosing cholangitis
Specific microbiome signature is found in primary sclerosing cholangitis
Veilonella is increased in human primary sclerosing cholangitis and other human chronic inflammatory disorders
Primary sclerosing cholangitis microbiome signature is different than the one observed in patients with UC without liver disease
Multidrug resistance 2-deficient germ-free mice exhibit more pronounced primary sclerosing cholangitis-like liver disease
Liver cirrhosis and microbiota
Liver regeneration
Besides many immune factors especially various cytokines such as IL-6, the gut microbiota might also affect and regulate liver regeneration. After antibiotic therapy, especially when using ampicillin, the number of CD1d-dependent natural killer T (NKT) cells was markedly reduced after partial hepatectomy. These NKT cells and activated Kupffer cells produced high amounts of the cytokines interferon-γ and IL-12 and neutralisation especially of IL-12 abrogated the negative effects of antibiotic therapy on liver regeneration. Antibiotic therapy after hepatectomy therefore could have a negative effect on liver regeneration potentially also in humans.66 Indeed, a recent study suggests that specific intestinal bacteria are closely associated with expression of certain genes in a regenerating liver.67 Partial hepatectomy resulted in an upregulation of more than 6000 bacterial microbiotal genes, some of them involved in bile acid metabolism and hepatocyte proliferation. This type of surgery was accompanied by massive changes in the IM, for example, an increase of Bacteroidetes and Rikenellaceae and a decrease in Clostridiales, Lachnospiraceae and Ruminococcaceae. It is well established that conventional inflammatory cytokines and Wnt factors control tissue repair and liver regeneration.68 Although sterile inflammation may play here the dominant role, it may well be that the gut microbiota acts as another driving force of this type of inflammation. Therefore, the gut microbiota seems to be a so far ignored player for successful liver regeneration.
Liver cirrhosis
Patients with liver cirrhosis show an increase in the translocation of intestinal bacteria and increased circulating concentrations of bacterial DNA suggesting that indeed the GI tract might reflect the major source of pathogens/pathobionts. Bacterial translocation plays a key role in the appearance of systemic infections in liver disorders and gut bacteria might differ in their ability to translocate or bypass the intestinal barrier. In gnotobiotic mice, enteric bacteria such as Escherichia coli, Proteus and Enterobacter are associated with a higher incidence of bacteraemia, because these bacteria translocate more efficiently from the GI tract than anaerobes. The faecal microbial community in patients with liver cirrhosis in comparison with healthy individuals had been characterised.69 In this study, the faecal microbial communities were analysed by 454 pyrosequencing of the 16S ribosomal RNA V3 region and by subsequent real-time qPCR. Bacteroidetes was significantly reduced, whereas Proteobacteria and Fusobacteria were highly increased in the cirrhosis group. Importantly, the prevalence of potentially pathogenic bacteria, such as Enterobacteriaceae and Streptococcaceae, with decreased presence of beneficial populations such as Lachnospiraceae might affect clinical phenotype and even prognosis in these patients. An important study recently assessed the gut microbiome in relation to cirrhosis severity, its stability over time and alterations with decompensation.70 Here, the authors used multitagged pyrosequencing and observed that progressive changes in the gut microbiome accompany cirrhosis and become more severe in case of decompensation. The ratio of autochthonous to non-autochthonous taxa was calculated as the cirrhosis dysbiosis ratio (CDR) and a low number indicated dysbiosis. CDR was highest in controls, lower in compensated and lowest in decompensated and inpatients and negatively correlated with endotoxin plasma levels. Importantly, in their study microbiota was significantly different between infected and non-infected cirrhotics. These authors recently showed that patients with liver cirrhosis exhibit a profound salivary dysbiosis.71 In this study, in 102 studied patients stool and saliva microbiome differed markedly on principal component analysis. The salivary microbiome with previously diagnosed hepatic encephalopathy (HE) showed an increase in pathogenic Enterobacteriacceae and Enterococcaceae. Changes in stool microbiota, however, correlated better with signs of systemic inflammation in these patients. The authors concluded that patients with liver cirrhosis exhibited both salivary and stool dysbiosis and changes were more pronounced in patients requiring the follow-up hospitalisations.
Qin et al investigated 98 patients with liver cirrhosis and 83 healthy controls using metagenomic analysis.72 They showed a profound dysbiosis and interestingly 54% of the patient-enriched taxonomically assigned species were of buccal origin, suggesting that the oral microbiota when present in the lower GI tract could contribute to disease process/severity. All these studies clearly support a major role for the intestinal microbiota in liver cirrhosis. At least in animal models the gut microbiota changes significantly in the progression of liver disease.73 Here, the authors induced liver disease by a streptozotocin high-fat diet leading to steatosis, fibrosis and finally hepatocellular carcinoma. Several bacterial species such as various Bacteroides, Atopobium spp, Clostridium cocleatum, Clostridium xylanolyticum and Desulfovibrio increased over disease progression and correlated with endotoxemia and clinical features. Liver cirrhosis can be considered as a prototypic microbiota-driven disorder (box 4).
Microbiota and liver cirrhosis
Infections remain the most common cause of death in these patients
Liver cirrhosis is associated with a profound dysbiosis; Bacteroidetes are significantly reduced, whereas Proteobacteria and Fusobacteria are highly increased in the cirrhosis group
Oral microbiota becomes highly present in the intestine
Advanced liver diseases and subsequent decompensation of liver cirrhosis is characterised by worsening of dysbiosis
Typical complications of advanced liver disease such as hepatic encephalopathy are substantially microbiota-driven
Gut-brain axis and microbiota in HE
A series of preclinical and some clinical studies have clearly established the bidirectional communication between the brain and the gut microbiota.74–76 Several communication channels by which gut microbes can signal to the brain have been identified, including neuronal, endocrine and immune-mediated pathways.77 The type and amount of gut microbial information that reaches the brain is greatly dependent on the regional intestinal milieu the microbes inhabit (influenced by regional intestinal transit, mucus secretion, production of antimicrobial peptides and intraluminal release of neuroactive substances such as noradrenaline), permeability of the gut epithelial barrier and the blood-brain barrier and the clearance of gut microbial metabolites through the liver, all of which are highly variable.74 ,78 Altered gut microbiome brain signalling, including the development of low-grade diet-induced ‘metabolic endotoxemia’ has been implicated in the pathophysiology of several psychiatric and neurological disorders,75 ,79 as well as in the pathophysiology of HE.80 HE is a severe neuropsychiatric complication of both acute and chronic liver failure. It is characterised by deficits in psychiatric, neurocognitive and motor functions, ranging from minimal HE including reversal of sleep-wake cycle, short-term memory loss, poor concentration and deficits that may only become apparent on formal neurocognitive testing to the most severe symptoms of delirium and coma.81–83
For more than 100 years, increased ammonia levels and their toxic effects on the brain have remained the most widely accepted mechanism underlying the development of HE,84 and various ammonia-lowering strategies (lowering of protein ingestion, broad-spectrum antibiotics, lactulose) remain the standard of care for HE today. Ammonia has long been thought to be produced primarily by urease producing gut microbiota (figure 3), but more recent evidence implicates an important role of the viscera (in particular small intestine and kidneys) as well.85 ,86 In addition to such increased generation of ammonia, the compromised clearance of portal venous ammonia by the cirrhotic liver and increased uptake of ammonia by astrocytes in the brain are thought to play important roles in development of neurological symptoms (figure 3). The original ammonia hypothesis has been expanded through the identification of additional synergistic factors including regional increases in GABAergic tone in certain brain regions,87–89 compromised intestinal barrier function, low-grade systemic inflammation, neuroinflammation80 and alterations in the gut microbiome.70 ,83 It is likely that many of these factors interact resulting in altered brain function. For example, compromised barrier function both at the gut and brain level can increase the amount of inflammatory and neuroactive metabolites that reach the brain. It is conceivable that under these conditions, GABA generated by certain gut microbes90 could reach the brain and contribute to the increased GABAergic tone observed in HE.87 Gut-generated inflammatory signals can reach the brain via different mechanisms, including the activation of intestinal and vagal afferent pathways, direct cytokine and LPS signalling through the bloodstream, facilitated by increased permeabilities of intestinal and blood-brain barriers and migration of primed monocytes through the blood-brain barrier (figure 3).80 Neuroinflammation results from regional activation of glial cells, the resident macrophages that make up the majority of cells in the brain, which upon stimulation generate a series of cytokines with effects on neuronal function and glutaminergic neurotransmission.
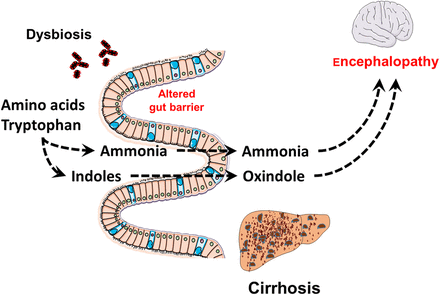
Gut-liver-brain axis and contribution to encephalopathy. Gut bacteria metabolise amino acids into specific metabolites including indoles and ammonia. Besides the altered gut barrier function, cirrhosis is also characterised by an alteration of the detoxification of ammonia, directly coming from the gut, as well as other specific compounds or derivatives such as oxindole that are by-products of indoles. Thus, among the different mechanisms linking metabolites of microbes and encephalopathy, several strong associations have been found between both ammonia and indoles/oxindole levels.
Direct evidence for the important role of the gut microbiota in the development of brain changes and cognitive dysfunction in HE comes from recent clinical and preclinical studies.91 ,92 In a comprehensive study in 147 cirrhotics and 40 healthy controls, Ahluwalia et al performed systemic inflammatory assessment, cognitive testing, assessment of regional brain astrocyte and metabolite changes by MR spectroscopy (MRS) and assessment of neuronal integrity and oedema by diffusion tensor imaging, 16S RNA sequencing of microbial composition and inference of the metagenomic content of samples from 16S data.83 As expected, patients with HE had greater impairment of cognitive function, more evidence for systemic inflammation, dysbiosis and hyperammonaemia compared with healthy controls and cirrhotics without HE. Cirrhotics with HE had a higher relative abundance of autochthonous taxa and a higher abundance of Staphylococcaceae, Enterococcaceae, Porphyromonadaceae and Lactobacillaceae compared with cirrhotics without HE and with healthy controls. Imputed stool microbial functionality in cirrhotics with HE was related to functions of endotoxin, endotoxin-protein synthesis and a shift towards an ammonia generating amino acid profile (figure 3). Intriguingly, specific microbial taxa (autochtonuous taxa negatively, Enterobacteriaceae positively) differentially correlated with hyperammonia-associated astrocytic changes on MRS (increased glutamate/glutamine ratios, reduced myoinositol). On the other hand, the taxa Porphyromonadaceae were only correlated with neuronal changes as seen on DTI of white matter integrity, but not with ammonia levels. These findings for the first time demonstrate correlations between altered gut microbial taxa and specific neuronal and astrocytic changes in the brain of cirrhotics with HE. Kang et al92 further characterised the relative role of the gut microbiota and ammonia levels in the development of neuroinflammation in a CCl4-induced model of cirrhosis in GF and in conventional mice. When compared with non-cirrhotic GF animals, cirrhotic GF mice showed higher ammonia levels, but no evidence of systemic or brain-related inflammatory changes. In contrast, conventional cirrhotic mice had intestinal dysbiosis as well as evidence for inflammatory brain changes. Similar correlations between gut microbial taxa, inflammatory marker and ammonia were identified in the earlier human study. Besides the role of ammonia, other data have shown that other products resulting from the metabolisation of amino acids such as tryptophan can also lead to the production of compounds such as indoles that can be further transformed into oxindole. For example, Riggio et al93 showed that blood levels of total indole compounds were higher in patients with HE (figure 3). They also found that oxindole levels were further higher in patients with liver cirrhosis. Interestingly, both ammonia and oxindole levels are positively correlated, thereby suggesting a similar gut-liver-brain axis in the physiopathology of HE. Another study by Montagnese et al94 also showed a strong association between ammonia and indole/oxindole levels and the alteration of EEG, a sign of neurophysiological abnormalities that can already be observed in more compensated patients (figure 3).
In summary, extensive preclinical and clinical evidence supports a crucial role of altered communication between the gut, its microbiota and the brain in the development of HE. A series of reported findings, including small intestinal overgrowth, gut dysbiosis, increased gut permeability, metabolic endotoxemia and brain changes (including regional neuroinflammation and possibly increased GABAergic tone) are consistent with this concept. These findings have important therapeutic implications. They provide a rationale for the traditional ammonia or indoles-lowering strategies, which have long been used in clinical management of HE. In addition, they point towards potential novel treatment strategies, including novel non-absorbable broad-spectrum antibiotics, and future microbial-specific antibiotics to avoid the common side effects of currently used antibiotics, towards drugs aimed at increased astrocyte activation in the brain (including minocycline), as well as different probiotic and prebiotic treatments.91 ,95 It is conceivable that the choice of therapeutic approach and the prediction of outcome will be influenced by the baseline assessment of gut microbial composition and metabolite profiles (box 5).
Gut-brain axis and microbiota
There are bidirectional communication channels between the gut, its microbiota and the brain
The trafficking in these channels is significantly altered in hepatic encephalopathy (HE) due to alterations in the gut microbiota and their metabolites, as well as to increased permeability of the epithelial barrier and the blood-brain barrier
In HE, both inflammatory signal as well as neuroactive microbial metabolites reach the brain and can induce regional neuroinflammation in the brain
Both structural and functional brain alterations have been reported in patients with HE, and these alterations show correlations with behavioural alterations and symptoms
Altered brain gut microbiome interactions in HE provide targets for novel treatment approaches, including prebiotics and probiotics, and microbe-specific antibiotics
Conclusions
Microbiome research in liver disease has evolved recently as an exciting new research field. Clinical but especially preclinical experimental studies have suggested that microbial factors are driving forces in many different liver diseases and at various stages of liver diseases. The microbiota affects rather diverse pathophysiological processes such as hepatic steatosis, liver inflammation, fibrosis development and HE. These studies have now convincingly established that the microbiota play a critical role in these diseases. Nevertheless, more studies are needed, especially metagenomic and metabolomic studies are eagerly expected to first of all provide respective descriptive data on how the gut microbes and their metabolites are altered in various clinical settings of liver disease. In a second and more comprehensive step, preclinical studies are mandatory to settle which microbiotal strains affect disease phenotype, that is, either acting protective or detrimental. In a further clinical step, clinical studies will be needed in different clinical situations to manipulate the microbiota by various strategies including either prebiotics, new probiotics or antibiotics or using FMT. It needs to be established in the future how manipulation of the gut microbiota might prove beneficial for the treatment of patients with various liver diseases at either early or later disease stages. Anyway, a new area has started in hepatology, which will attract many scientists and physicians to investigate all these new excitements.
References
Footnotes
Twitter Follow Patrice Cani at @MicrObesity
Funding HT is supported by the excellence initiative (Competence Centers for Excellent Technologies) of the Austrian Research Promotion Agency (FFG): Research Center of Excellence in Vascular Ageing—Tyrol, VASCage (K-Project Nr. 843536) funded by the BMVIT, BMWFW, the Wirtschaftsagentur Wien and the Standortagentur Tirol. PDC is a research associate at FRS-FNRS (Fonds de la Recherche Scientifique), Belgium. PDC is the recipient of grants from FNRS (J.0084.15) and ARC (Action de Recherche Concertée—Communauté française de Belgique convention: 12/17-047). This work was supported by WELBIO under grant: WELBIO-CR-2012S-02R, and the Funds Baillet Latour (Grant for Medical Research 2015). PDC is a recipient of an ERC Starting Grant 2013 (European Research Council, starting grant 336452-ENIGMO).
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.