Article Text
Abstract
BACKGROUND Balsalazide is a new 5-aminosalicylic acid (5-ASA) containing prodrug. Its efficacy in comparison with standard mesalazine therapy and the optimum dose for maintaining remission of ulcerative colitis are still unclear.
AIMS To compare the relapse preventing effect and safety profile of two doses of balsalazide and a standard dose of Eudragit coated mesalazine.
METHODS A total of 133 patients with ulcerative colitis in remission were recruited to participate in a double blind, multicentre, randomised trial: 49 patients received balsalazide 1.5 g twice daily, 40 received balsalazide 3.0 g twice daily, and 44 received mesalazine 0.5 g three times daily. Efficacy assessments were clinical activity index (CAI) and endoscopic score according to Rachmilewitz, and a histological score. In addition, laboratory tests were performed and urinary excretion of 5-ASA and its metaboliteN-Ac-5-ASA was analysed. The study lasted for 26 weeks.
RESULTS Balsalazide 3.0 g twice daily resulted in a significantly higher clinical remission rate (77.5%) than balsalazide 1.5 g twice daily (43.8%) and mesalazine 0.5 g three times daily (56.8%) (p=0.006). The respective times to relapse were 161 days, 131 days (p=0.003), and 144 days (NS). Accordingly, pairwise contrasts of the final endoscopic score demonstrated a significant difference (p=0.005) between the two balsalazide treatment groups while differences between either of these two groups and mesalazine were not statistically significant. Patients treated with balsalazide excreted less 5-ASA andN-Ac-5-ASA than patients receiving mesalazine but these differences were not statistically significant. Discontinuation of the trial because of adverse effects occurred in nine patients: three in the balsalazide 1.5 g twice daily group, two in the balsalazide 3.0 g twice daily group, and four in the mesalazine 0.5 g three times daily group. No clinically important new drug safety related findings were identified in this study.
CONCLUSIONS High dose balsalazide (3.0 g twice daily) was superior in maintaining remission in patients with ulcerative colitis compared with a low dose (1.5 g twice daily) or a standard dose of mesalazine (0.5 g three times daily). All three treatments were safe and well tolerated.
- balsalazide
- mesalazine
- aminosalicylic acid
- ulcerative colitis
Abbreviations used in this paper
- 5-ASA
- 5-aminosalicylic acid
- UC
- ulcerative colitis
- 4-ABA
- 4-aminobenzoyl-β-alanine
- SASP
- sulphasalazine
- N-Ac-5-ASA
- 5-N-acetylaminosalicylic acid
- CAI
- clinical activity index
- SDS-PAGE
- sodium dodecyl sulphate-polyacrylamide gel electrophoresis
Statistics from Altmetric.com
Treatment with 5-aminosalicylic acid (5-ASA) containing drugs is the gold standard for maintaining remission in patients with ulcerative colitis (UC). In order to deliver orally administered 5-ASA to the inflamed colonic mucosa where it exerts its topical action, two different modes of targeting have been devised: 5-ASA linked to a carrier molecule (“prodrug”) preventing absorption in the small intestine or specially coated 5-ASA (“mesalazine”) forming a delayed release preparation.
Balsalazide is a novel prodrug where 5-ASA is azo bound to 4-aminobenzoyl-β-alanine (4-ABA). The azo bond is split by colonic bacteria to release 5-ASA in the colon to exert its anti-inflammatory action. A significant difference between balsalazide and currently used prodrugs (for example, sulphasalazine, olsalazine) is that both the carrier molecule 4-ABA and the prodrug are pharmacologically inert and devoid of systemic effects.1
A recent meta-analysis2 comparing the relapse preventing effects of the prodrug sulphasalazine (SASP) compared with sulpha free 5-ASA preparations demonstrated the therapeutic superiority of SASP. 5-ASA compounds also included mesalazine and prodrugs. Nevertheless, because of their favourable tolerability and safety, there is a clear tendency towards treatment with sulpha free 5-ASA preparations in chronic UC. A trial comparing a prodrug (olsalazine) with mesalazine for maintenance therapy showed better therapeutic effects with the prodrug.3
Comparative trials have established that balsalazide is as effective as SASP, and better tolerated in both active and quiescent UC.1 However, there are only limited data comparing balsalazide and mesalazine. We present the results of a trial comparing the therapeutic effects and tolerability of balsalazide with a standard dose of mesalazine for maintenance therapy of UC. In addition, we tested if there is dose dependency for the effects of balsalazide. In contrast with the situation in active UC, it is unclear if an increase in the dose of the respective 5-ASA compound improves maintenance of remission in patients with UC.4
Materials and methods
PATIENTS
Twenty one centres in Germany enrolled a total of 133 patients (fig 1). The clinical phase lasted from February 1994 to September 1997. Patients gave written informed consent and approval of the ethics committee of the Ärztekammer Nordrhein as well as of the local research ethics committees were obtained. Inclusion criteria were: UC involving at least the rectum and sigmoid colon; UC in remission for less than one year; at least two acute attacks of UC in the medical history; remission—clinical as well as endoscopic; and age between 18 and 70 years. Specific exclusion criteria were: proctitis without further extent of the disease; treatment with oral, intravenous, or rectal steroids within 14 days prior to visit 1; use of antibiotics within 14 days prior to visit 1 except for short term therapy of a defined infection; immunosuppressive therapy within the last three months; regular treatment with non-steroidal anti-inflammatory agents; and intolerance to 5-ASA.
Disposition of the patients enrolled in the study.
METHODS
The study was a randomised, multicentre, double blind, double dummy, three armed, parallel group comparison of balsalazide 1.5 g twice daily, balsalazide 3.0 g twice daily, and mesalazine 0.5 g three times daily for 26 weeks. No UC medication other than the respective study preparations was allowed throughout the trial.
Balsalazide was supplied as capsules containing 0.75 g balsalazide (manufactured by Salix, USA). Mesalazine was supplied as tablets containing 0.5 g 5-ASA coated with Eudragit L (Salofalk; manufactured by Dr Falk Pharma GmbH, Germany). Placebos of identical appearance to balsalazide capsules were manufactured by Salix and to mesalazine tablets by Astra GmbH (Germany). Balsalazide 1.5 g twice daily corresponds to a total dose of 1.05 g 5-ASA/day, balsalazide 3.0 g twice daily corresponds to 2.10 g 5-ASA/day, and mesalazine 0.5 g three times daily corresponds to 1.50 g 5-ASA/day.
STUDY OBJECTIVES
The primary objective was to investigate the efficacy of balsalazide in maintaining clinical remission in UC compared with a mesalazine preparation with pH dependent release of 5-ASA. The secondary objective was to investigate the systemic effects of balsalazide compared with mesalazine, especially the influence on renal function.
CLINICAL ASSESSMENTS
At entry, the patient's medical history was recorded and a physical examination was performed. A total colonoscopy was performed within 10 days prior to intake of the first dose of the investigational medication. Sigmoidoscopy was performed at the discretion of the investigator if colonoscopy had been performed during the last six months prior to entry into the trial. The patient's overall evaluation of symptoms was assessed according to the clinical activity index (CAI) of Rachmilewitz.5 Clinical remission was defined as a CAI <6. Endoscopic findings were also classified according to Rachmilewitz5 and an EI score <4 was considered to indicate remission. Relapse was defined as CAI ⩾6 and EI >4. The primary variable of the study was “remission of UC”, defined as clinical as well as endoscopic remission at completion of the study. In addition, biopsies from the rectum and sigmoid colon were taken at entry and at the end of the study period and evaluated by a single pathologist (MS). Classification of histological activity was performed according to Truelove and Richards,6 whereas mild and moderate activity were classified separately. The following classes were used: 1, normal mucosa; 2, UC in remission; 3, UC with mild activity; 4, UC with moderate activity; 5, UC with severe activity; and 6, no UC.
LABORATORY ASSESSMENTS
Routine haematology, clinical chemistry, and urine analysis were performed throughout the study. At entry and at the end of the study, additional urine analyses were undertaken. Twenty four hour urine was collected within five days prior to administration of the first dose of the investigational drug. At the end of the study, urine collection was started within 10 days prior to intake of the last dose of the investigational drug. The volume of urine was measured and a portion was labelled and frozen at −20°C. In addition, a portion of morning urine was labelled and frozen.
Analysis of 5-ASA and its metabolite 5-N-acetylaminosalicylic acid (N-Ac-5-ASA) in the 24 hour urine collection was performed using a standardised high pressure liquid chromatography.7 Urine samples were subjected to deproteinisation with acetonitrile, subsequent derivatisation of 5-ASA, and liquid-liquid extraction with ethylacetate. Chromatography was carried out on a Nucleosil 10 SB column with fluorescence detection. The calibration range of the method was fixed at approximately 50–2000 ng/ml 5-ASA and 100–4000 ng/ml N-Ac-5-ASA. Determinations were performed in duplicate and the mean value calculated. The 24 hour urine collection was also analysed for glutathione-S-transferase-π. Morning urine was subjected to protein analysis by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).8 ,9 The lower sensitivity limit for protein detection with this method is 5 mg/l. The analysis allows differentiation between glomerular and tubular proteinuria. The results were scored as “no proteinuria” (negative) and “tubular proteinuria” (positive).
CLINICAL VISITS AND DIARY CARD ASSESSMENTS
Clinic visits took place at entry and on completion of the study, and at weeks 4, 8, and 17. A physical examination was performed at each visit. At the first visit or at unscheduled visits, retrospective data for number of stools, blood, abdominal pain, and fever were used. At all other visits, patients completed a diary card daily, noting these items during the week prior to the clinical visit. Extraintestinal manifestations as well as the type and severity of adverse events were assessed by the investigator on the day of the clinic visit. Laboratory findings originated from the screening programme performed on the day of the clinic visit. To monitor compliance with treatment, at each clinic visit patients were asked to return investigational drugs, and the amount of drug remaining was checked.
STATISTICAL ANALYSIS
Analyses of all patients treated (intent to treat analysis) were performed (fig 1). The proportion of patients who were still in remission at the end of the study was calculated for each treatment group. A χ2 test with two degrees of freedom was then used to assess the evidence that there was a difference in the remission rates of the three treatment groups. A χ2 test with one degree of freedom was used to compare remission rates of the treatment groups pairwise. The last value extended principle was used for symptom assessment, provided the patient had at least one assessment after entry. To detect a difference in remission rates of 25%, assuming a remission rate of 85% under balsalazide and 60% under mesalazine, it was estimated that 186 patients with 62 patients per group had to be enrolled into the study (α=0.05; 1−β=0.9). According to existing data, no difference in efficacy between the low and high balsalazide doses was expected.
Time until relapse was assumed to be time until discontinuation or relapse and was censored for those patients still in remission at the end of the study. A log rank test was used to compare time until relapse between treatment groups, and Kaplan-Meier estimates were calculated and plotted.
CAI, endoscopic, and histological scores at the patient's last visit were analysed using an ANOVA model.
Using the treatment means obtained from analysis of variance, the slope and intercept (and their error structure) of the dose-response line, on the log dose scale, for the two balsalazide doses was calculated together with the dose of balsalazide (per day) which was equivalent to mesalazine 0.5 g three times daily. Using Fieller's theorem, 95% confidence intervals for this equivalent dose were determined. These 95% confidence intervals were determined for CAI, endoscopic, and histological scores.
Results
A total of 132 patients were eligible for the intent to treat analysis (fig 1). Treatment groups were comparable at randomisation for baseline demographics (table 1) and UC history (table 2). A total of 92 patients completed the study. Reasons for discontinuation are given in table3.
Patient characteristics in the balsalazide twice daily (bid) and mesalazine three times daily (tid) groups at entry
Patient history of ulcerative colitis, symptoms, and endoscopic examination in the balsalazide twice daily (bid) and mesalazine three times daily (tid) groups at entry
Patients discontinuing the study in the balsalazide twice daily (bid) and mesalazine three times daily (tid) groups
CLINICAL EFFICACY
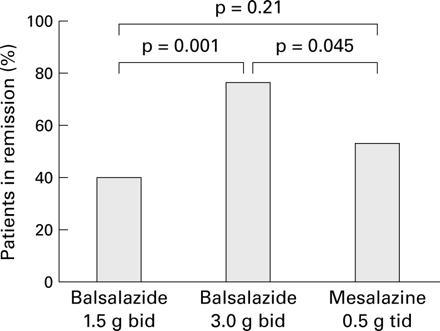
The percentage of patients still in remission after 26 weeks is shown in fig 2. There was significant evidence that the three treatment groups did not have the same remission rate (χ2 test; p=0.006). The 3.0 g twice daily dose of balsalazide had a higher (p=0.001) remission rate (77.5%) than the 1.5 g twice daily dose of balsalazide (43.8%). The balsalazide 3.0 g twice daily group also had a higher (p=0.045) remission rate than the 0.5 g three times daily mesalazine group (77.5% compared with 56.8%). There was no statistically significant difference between the mesalazine and low dose balsalazide group (p=0.21).
Percentage of patients still in remission after 26 weeks of treatment with balsalazide twice daily (bid) or mesalazine three times daily (tid) (χ2 tests).
Differences between time to relapse in the three groups were found to be statistically significant (log rank test, p=0.01). Kaplan-Meier estimates for time to relapse are plotted in fig 3. Pairwise contrasts between treatment groups showed a significant (p=0.003) difference between the high and low dose balsalazide groups (time to relapse 161 days compared with 131 days) while there were no significant differences between the high dose balsalazide group and the mesalazine group (time to relapse 144 days), or between the low dose balsalazide and mesalazine groups.
Kaplan-Meier estimates for time in remission for patients treated with balsalazide twice daily (bid) or mesalazine three times daily (tid).
Pairwise contrasts of the final CAI again showed a significant difference (χ2 test; p=0.008) between the high and low dose balsalazide groups whereas differences between the balsalazide groups and the mesalazine group did not reach significance (table 4). In addition, baseline CAI was a significant (p=0.023) covariate in the analysis of the final CAIs—that is, the final CAI value was dependent on CAI at the start of the study.
Pairwise contrasts of the final clinical activity index (CAI), endoscopic, and histological scores in the balsalazide twice daily (bid) and mesalazine three times daily (tid) groups
Using CAI data, the dose of balsalazide equivalent to mesalazine 0.5 g three times daily was estimated to be 2.13 g twice daily (95% confidence interval 1.16; 4.19) (fig 4). A dose of 2.13 g twice daily balsalazide corresponds chemically to 1.49 g/day 5-ASA which is very similar to 1.50 g/day 5-ASA for 0.5 g three times daily mesalazine. Thus it can be assumed that a balsalazide dose comparable with that of mesalazine with an equivalent amount of 5-ASA had similar efficacy.
Estimated treatment effects and 95% confidence intervals for equivalent dose of mesalazine with respect to balsalazide, using a clinical activity index.
ENDOSCOPIC ASSESSMENTS
Pairwise contrasts of the final endoscopic score demonstrated a statistically significant difference (p=0.005) between the two balsalazide treatment groups. There was no evidence of a difference in the last visit endoscopic score for mesalazine compared with the two balsalazide treatments (table 4). Baseline endoscopic score was not a significant covariate (p=0.133)—that is, the final endoscopic score for a patient was not correlated with endoscopic score at visit 1.
HISTOLOGICAL ASSESSMENTS
Pairwise contrasts of the final histological score demonstrated no significant differences (table 4).
URINE DATA
Amounts of 5-ASA excreted as 5-ASA andN-Ac-5-ASA in urine were calculated as a percentage of 5-ASA dose, and the two balsalazide groups combined, as described in statistical analysis. Excretion and recovery are shown in table 5. Although patients treated with balsalazide excreted less amounts than patients who received mesalazine, there was no significant difference either in 5-ASA excretion (χ2 test p=0.147) orN-Ac-5-ASA excretion (p=0.147), or in recovery rate (p=0.124).
Urinary 5-aminosalicylic acid (5-ASA) and 5-N-acetylaminosalicylic acid (N-Ac-5-ASA) excretion and recovery of 5-ASA taken by mouth (% of the 5-ASA dose): database on 24 hour urine collection
Urine proteins were assessed using SDS-PAGE. A positive result was defined as a protein pattern indicative of tubular damage whereas a negative result reflected normal findings. The data are listed in table6. There was no evidence of any difference between groups in the proportion of patients with negative results at the beginning of the study and positive results at the end (p=0.99).
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) at the start and end of the study in the balsalazide twice daily (bid) and mesalazine three times daily (tid) groups
Glutathione-S-transferase-π was measured to assess tubular damage. There were few positive measurements. Changes in enzyme levels from the beginning to the end of the study were compared between the three groups using the Kruskal-Wallis test. There was no evidence of any difference between the three groups (p=0.49).
SAFETY EVALUATION
All patients who took at least one dose of the randomised investigational medication were included in the analyses (fig 1). As listed in table 3, nine patients discontinued the study because of adverse events. Reasons for premature termination of the study were headache, hypertension, malaise, dizziness, abdominal pain, pruritus, and skin rash (three patients, more than one event per patient) in the balsalazide 1.5 g twice daily group, pancreatitis, gingivitis, alopecia, and nail disorders (two patients) in the balsalazide 3.0 g twice daily group, and palpitation, hypotension, tenesmus, nausea, impotence, diarrhoea, and alopecia (four patients) in the mesalazine 0.5 g three times daily group.
Overall, no clinically important new drug safety related findings were observed. The percentage of patients reporting any adverse event, including laboratory findings, was 38% in the balsalazide 1.5 g twice daily group, 53% in the balsalazide 3 g twice daily group, and 45% in the mesalazine 0.5 g three times daily group. Figure 5 depicts the number of adverse events per 1000 treatment days, presented by system organ class.
Adverse events by system organ class (SOC): number of adverse events per 1000 treatment days after balsalazide twice daily (bid) or mesalazine three times daily (tid). RES, reticuloendothelial system.
STABILITY OF RESULTS
Analysis of remission rate was repeated, considering only patients who completed the study according to the protocol (per protocol analysis). The number of patients and respective remission rates were: balsalazide 1.5 g twice daily (n=42; 45%); balsalazide 3.0 g twice daily (n=38; 79%); mesalazine 0.5 g three times daily (n=40; 60%). Thus the results of the intent to treat analysis were confirmed.
Similarly, a per protocol analysis of time until relapse was performed. The conclusions of this analysis were the same as those for the intent to treat analysis. Also, conclusions concerning endoscopic treatment effects were qualitatively unchanged.
Discussion
We have demonstrated the striking superiority of a high dose of balsalazide over a lower dose of balsalazide in maintaining remission in UC. This is of interest as results on the dose dependency of maintenance therapy have to date been either conflicting or suggest that the benefits of high doses are modest.4 Azad Khanet al were the first to perform a dose finding study for maintenance treatment in UC.10 They compared three doses of SASP, 1 g/day, 2 g/day, and 4 g/day, and found relapse rates of 33%, 14%, and 9%, respectively. Apparently, these data cannot confirm a linear dose relationship but rather indicate a threshold dose for clinical efficacy. Relatively few studies have assessed the value of high dose 5-ASA treatment,4 and a recent meta-analysis showed no clear evidence of a dose-response effect.2 Indeed, the rate for maintaining remission of 43.8% for 1.5 g twice daily balsalazide is within the range of placebo effects in four other studies.2 But the placebo results2 showed considerable variability, some clearly inferior to the effects of balsalazide 1.5 g twice daily, indicating different patient populations, making this type of comparison impossible.
Two other studies have compared the effects of balsalazide in preventing relapse. A dose of 4 g/day was found to be superior to 2 g/day11 but doses of 3 g and 6 g daily seemed to be equivalent.12 The study population in the latter trial12 differed in some aspects from that in our investigation: patients were included with a very distal extent of UC, and a major proportion of patients had longstanding remission (>1 year). Thus our results are in line with the suggestion that patients with a more extended UC or with frequent relapses may benefit from a higher dose of maintenance therapy.4
For historical reasons, most trials that have compared the therapeutic effects of different aminosalicylates involved SASP. A meta-analysis showed significant superiority of SASP over sulpha free 5-ASA compounds in maintaining remission in UC.2 It is of interest that the meta-analysis2 comprised six studies with mesalazine, of which three showed a trend towards better maintenance efficacy than SASP whereas the prodrugs investigated (five olsalazine, one balsalazide) all performed worse than SASP. With respect to the action of 5-ASA, SASP can be considered as a prodrug but on the other hand the unsplit double molecule SASP as well as the carrier molecule sulphapyridine both have beneficial and adverse effects. Therefore, it remains unclear whether the therapeutic superiority of SASP2 can be attributed to its prodrug characteristic or to its specific pharmacological properties. As yet, there are only two published maintenance trials comparing different 5-ASA drugs.3 ,13 Comparisons of the relapse preventing capacity of Eudragit S coated mesalazine with that of the two prodrugs olsalazine and balsalazide showed (for 12 months) significant therapeutic superiority of olsalazine3 and similar efficacy of balsalazide,13 respectively.
It was suggested3 that the beneficial effects of the prodrug were mainly due to its therapeutic superiority in left sided colitis. Most interestingly, in the two published comparative trials in acute UC (mild to moderately active) this superiority of a prodrug over mesalazine in patients with left sided colitis was also observed.14 ,15 In the study presented here, 32.5% of patients had left sided colitis, a proportion lower than that in other comparative studies.3 Thus a potential therapeutic advantage of balsalazide based on its effects in left sided colitis may not have been evident.
In summary, taking into consideration corresponding 5-ASA amounts, balsalazide and Eudragit L coated mesalazine showed similar effectiveness in maintaining remission in UC.
Safety is a particularly important aspect in chronic maintenance treatment. Regarding the number and severity of adverse events, our study showed no clinically relevant differences either between the high and low doses of balsalazide or between balsalazide and mesalazine. No unexpected adverse events were observed. To date, the preparations investigated here confirm general experiences with aminosalicylates in the treatment of patients with inflammatory bowel disease—that is, safe and well tolerated.16 There is special interest regarding the putative nephrotoxicity of aminosalicylates. This toxic reaction may be acute (allergy) or chronic in a dose dependent manner.17 ,18 Therefore, urinary excretion of 5-ASA and its metabolite N-Ac-5-ASA was measured. In line with pharmacological studies,1 ,19 even during chronic treatment with a prodrug (balsalazide), excretion rates were lower than with mesalazine. Additional investigations (SDS-PAGE, glutathione-S-transferase-π activity) did not indicate tubular damage. Thus this study was unable to demonstrate any specific adverse reactions of balsalazide or mesalazine, with low or high doses, although balsalazide may have some advantages because of its lower urinary excretion rate of 5-ASA and its metabolite.
In conclusion, an equivalent dose of 5-ASA given as balsalazide or Eudragit L-coated mesalazine had comparable clinical efficacy in the maintenance therapy of UC. Relapse prevention can be significantly improved with a high dose of balsalazide. Both treatments, irrespective of the dose, were safe and well tolerated. In patients at risk (more extended disease, frequent relapses), high dose maintenance therapy should be considered.
Acknowledgments
The study was supported by Astra Zeneca GmbH Wedel, Germany. We would like to express our thanks to L Sallhoff for secretarial support. The study was presented in part at the Digestive Disease Week 1998 and published as an abstract in Gastroenterology1998;114:1014.
Conflict of interest. Dr Pallant and U Ewald were previously employed by Astra Draco (now Astra Zeneca).
Abbreviations used in this paper
- 5-ASA
- 5-aminosalicylic acid
- UC
- ulcerative colitis
- 4-ABA
- 4-aminobenzoyl-β-alanine
- SASP
- sulphasalazine
- N-Ac-5-ASA
- 5-N-acetylaminosalicylic acid
- CAI
- clinical activity index
- SDS-PAGE
- sodium dodecyl sulphate-polyacrylamide gel electrophoresis