Article Text
Abstract
Objective: The aim of the study was to compare sequential versus combined diuretic therapy in patients with cirrhosis, moderate ascites and without renal failure.
Design: One hundred patients were randomly assigned to the two diuretic treatments. The sequential treatment provided potassium canrenoate at the initial dose of 200 mg/day, then increased to 400 mg/day. Non-responders were treated with 400 mg/day of potassium canrenoate and furosemide at an initial dose of 50 mg/day, then increased to 150 mg/day. The combined treatment provided the initial dose of 200 mg/day of potassium canrenoate and 50 mg/day of furosemide, then increased to 400 mg/day and 150 mg/day, respectively.
Results: Most patients who received sequential treatment responded to potassium canrenoate alone (19% to 200 mg/day and 52.63% to 400 mg/day, respectively). Most patients who received the combined treatment responded to the first two steps (40% to the first step and 50% to the second, ie, 400 mg/day of potassium canrenoate plus 100 mg/day of furosemide). Adverse effects (38% vs 20%, p<0.05), in particular, hyperkalaemia (18% vs 4%, p<0.05), were more frequent in patients who received sequential therapy. As a consequence, the per cent of patients who resolved ascites without changing the effective diuretic step was higher in those who received the combined treatment (56% vs 76%, p<0.05).
Conclusions: The combined diuretic treatment is preferable to the sequential one in the treatment of moderate ascites in patients with cirrhosis and without renal failure.
NCT00741663. This work is an open randomised clinical trial.
Statistics from Altmetric.com
Ascites represents the most frequent complication of cirrhosis since it develops in more than 50% of patients within 10 years from the diagnosis of cirrhosis.1 Ascites is associated with a poor survival rate: 50% and 20% within 1 and 5 years, respectively.1 While paracentesis is the first choice treatment of massive ascites, moderate ascites should be treated with salt restriction and diuretics in order to create a negative sodium balance.2 3 Since a spontaneous resolution of ascites can be obtained simply by reducing the sodium content of the diet only in 10–20% of cases, a negative sodium balance can not be achieved without the use of diuretics in most patients with cirrhosis and ascites. Having found that aldosterone antagonists represent the first-line diuretics in the treatment of ascites in cirrhosis,4 two different schedules are being used in clinical practice. The first consists of the administration of increasing doses of spironolactone, adding furosemide only to those patients who did not respond to the high recommended doses of aldosterone antagonist (sequential diuretic treatment). The second provides the simultaneous administration of an aldosterone antagonist and loop diuretic from the beginning of the treatment, increasing the dose of both diuretics if no response is achieved (combined diuretic treatment).3 5 Up to now only one controlled clinical trial has compared these two schedules of diuretic treatment of ascites. In 2003 Santos et al showed that spironolactone alone was as effective as spironolactone plus furosemide. In addition, spironolactone alone induced an excessive diuretic response less frequently as compared to spironolactone plus furosemide. As a consequence, the authors concluded that sequential diuretic treatment represents the more suitable schedule for treating ascites in these patients.6 Nevertheless, it is worth observing that the study by Santos et al included a large proportion of previously untreated patients with cirrhosis and ascites, who are not commonly seen at tertiary referral centres. This is the main reason why in our hands7 and in the hands of other authors8 spironolactone alone is effective in mobilising ascites in only 56% and 77% of non-azotaemic patients with cirrhosis, respectively. As a result, a significant proportion of patients with cirrhosis and ascites should experience further steps of sequential diuretic treatment, prolonging both the time to achieve the effective step and, overall, the time to achieve the mobilisation of ascites.7 8 Therefore, an open randomised clinical study was performed by comparing sequential diuretic therapy versus combined diuretic therapy in the treatment of moderate ascites in non-azotaemic patients with cirrhosis as regards their efficacy and safety.
Materials and methods
Patients
All non-azotaemic patients with cirrhosis and ascites who were admitted to the four hospitals involved or evaluated as outpatients from April 2005 to September 2008 for the treatment of moderate ascites were considered for the study. The inclusion criteria were the following: (1) grade 2 ascites, (2) serum creatinine less than 1.2 mg/dl, (3) serum sodium >130 mEq/l, and (4) serum potassium within 3.5 and 4.5 mEq/l, at least 5 days after the withdrawal of diuretics and a 90 mmol/day Na diet. Exclusion criteria were the following: (1) any therapeutic paracentesis for ascites before inclusion, (2) cardiac or respiratory disease, (3) gastrointestinal bleeding, hepatic encephalopathy, bacterial infections in the 4 weeks before inclusion, and (4) the use of non-steroidal anti-inflammatory drugs (NSAIDs) or nephrotoxic drugs in the 4 weeks before inclusion. Written informed consent was obtained from the patients. The protocol conformed to the Declaration of Helsinki and Guidelines for Good Clinical Practice in Clinical Trials.
Baseline evaluations
Diuretics were withdrawn for at least 5 days after inclusion into the study. Patients were prescribed a 90 mmol/day Na diet throughout the study. On day 4, no beverage containing methylxanthine was allowed and at 9 p.m, 600 mg of lithium carbonate was administered. On day 5, after a 12 h fast, lithium clearance (C.Li), p-aminohippurate (PAH) clearance (C.PAH) and inulin (INU) clearance were evaluated as previously described.9 10 After a water load of 20 ml/kg of body weight and an initial 60 min equilibration period, urine samples were collected for one 4 h clearance period by spontaneous voiding. Blood samples were collected at the beginning, the midpoint and the end of the clearance period and analysed for plasma PAH, INU, serum urea, serum creatinine, serum Na, K, Li, serum concentration of nitrates and nitrites (NOx), plasma renin activity (PRA), and plasma concentration of aldosterone and vasopressin. Urine samples were analysed for PAH, INU, creatinine, Na, and Li. C.PAH and C.INU were used as measures of renal plasma flow (RPF) and glomerular filtration rate (GFR), respectively.9 10 C.Li was used as a measure of the delivery of fluid to the distal tubule.9 10
Protocol
On day 5, patients who fulfilled the inclusion criteria were randomised to receive sequential (Group A) or combined diuretic treatment (Group B). Randomisation was performed using consecutively numbered, computer-generated envelopes containing the treatment assigned in sets of 30 for each hospital. Randomisation was independent for each hospital. Treatment was started in the same day after randomisation.
Sequential and combined diuretic treatments are showed in fig 1. In Group A diuretic treatment was started with 200 mg/day of potassium canrenoate orally. The dose was increased to 400 mg/day if no response ensued. Patients who did not begin to mobilise ascites with potassium canrenoate alone were then treated with potassium canrenoate at the fixed dose of 400 mg/day and furosemide. Since in Italy, furosemide is available for oral administration at the minimal dose of 25 mg, the initial dose of furosemide was 25 mg, twice daily, orally. The dose of furosemide was then increased to 50 mg, twice daily, and 75 mg, twice daily, if no response was observed. In Group B, the diuretic treatment was started with 200 mg once daily of potassium canrenoate and 25 mg, twice daily, of furosemide orally. The doses of diuretics were gradually increased to 400 mg/day and 50 mg/day, twice daily, to 400 mg/day and 75 mg, twice daily, respectively, if no response was observed.
The schedule of diuretic treatment in patients who received sequential (Group A) or combined diuretic treatment (Group B).
The primary end-point of the study was the time to achieve an effective diuretic step. The secondary end point was a “failure” end point as defined by the development of diuretic-induced adverse effects prior to or after the achievement of an effective diuretic step. According to the guidelines on the use of diuretics in patients with cirrhosis and ascites,2 3 a body weight loss greater than 700 g every 3 days was considered to be an effective response to the step of diuretic therapy. Consequently, the step of diuretic therapy was upgraded every fourth day on the basis of no response. Excessive diuretic response was defined as a mean loss of body weight greater than 500 g/day in patients without peripheral oedema and greater than 1000 g/day in those with peripheral oedema evaluated each time on the basis of a 3 day clinical monitoring. To detect adverse effects, serum urea, creatinine, Na and K were evaluated every 3 days until almost complete mobilisation of ascites was achieved. Hyperkalaemia and hypokalaemia were defined as a serum K>5.5 mEq/l and <3.5 mEq/l in two consecutive serum samples, respectively. Hyponatraemia was defined by a serum Na less than 130 mEq/l and less than 125 mEq/l in two consecutive serum samples according to a baseline serum Na > or <135 mEq/l, respectively. Renal failure was defined when serum creatinine increased by more than 50% of the baseline value to levels higher than >1.5 mg/dl in the absence of other precipitating events (ie, bacterial infections). Hepatic encephalopathy was monitored by careful daily physical examination. Refractory ascites was defined according to the criteria of the International Ascites Club.3 No treatment was provided in order to prevent the development of adverse effects. In particular, potassium chelant and/or human albumin were not used in these patients in any part of the study. Patients who did not reach an effective diuretic therapy or developed either an excessive response or developed adverse effects to diuretics were considered to be treatment failures and therefore received individualised treatment according to the guidelines of the treatment in patients with cirrhosis and ascites.2 3 No drug known to affect renal function besides diuretics was prescribed during the study.
After the diuretic protocol study all included patients were checked every 2 weeks during the first month and then every 4 weeks for 5 months.
Analytical methods
Brachial artery pressure was taken with a standard mercury sphygmomanometer. Serum Na, K and Li, and urinary Na and Li were measured by a flame photometer (Instrumentation Laboratory model 143; Instrumentation Laboratory, Paderno Dugnano, Italy). Serum and urinary creatinine, plasma and urinary PAH and INU were evaluated colorimetrically, as previously reported.9 10 PRA was determined by radioimmunoassay (RIA) for angiotensin (Radim, Pomezia, Italy), as well as the plasma concentration of aldosterone (Technogenetics Group Bouty, Sesto San Giovanni, Italy), vasopressin (Diagnostic System Laboratories, Webster, Texas, USA). NOx were evaluated colorimetrically, as previously reported (Cayman Chemical Company, Milan, Italy).10
Calculations
Mean arterial pressure was calculated as the diastolic pressure plus one-third of the pulse pressure. Plasma Li was calculated on the basis of the correspondent values at the beginning and the end of the urine collection period according to the formula proposed by Thomsen and Leyssac.11 Clearance of creatinine, Na, Li, PAH and INU were calculated by the conventional formula Ca = (Ua × V)/Pa, where U and P are the urinary or plasma concentration of a, respectively, and V is the urinary output. Fractional urinary excretion of Na (FE Na) and Li (FE Li) and the delivery of sodium to the distal tubule were calculated as previously reported.9 10
Statistical analysis
For the calculation of the sample size it was hypothesised, on the basis of a previous study12 in which 50% of patients responded to 7 days of 200 mg/day of potassium canrenoate, and pharmacodynamic data on furosemide in patients with cirrhosis and ascites,13 that 30% and 60% of patients in Groups A and B, respectively, would reach the effective diuretic step during the first 3 days of treatment. Forty-nine patients had to be included in each group to obtain a p value <0.05 with an α error of 5% and a β error of 20%. Results are presented as means with the SD. A comparison between groups was performed with the use of the χ2 test or Fisher’s exact test for categorical data and the Student t test for continuous data. For the categorical variables the log-rank test of equality across strata was used. A p value <0.05 was accepted as being statistically significant.
Results
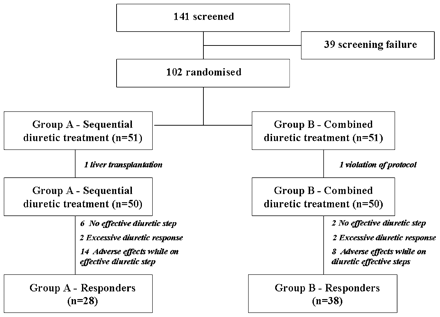
As reported in fig 2, a total of 141 patients with cirrhosis and ascites were screened. Thirty-seven patients were not included for the following reasons: 17 patients had a bacterial infection, six patients required therapeutic paracentesis for massive ascites, eight patients had an impaired renal function, three patients had hepatic encephalophaty, three had a serum sodium <130 mEq/l before inclusion. In addition, two patients refused to participate in the study. The final analysis of the trial included 100 patients (50 in the Padova hospital, 20 in the Cittadella hospital, and 15 in each of the two Treviso hospitals). Fifty patients were randomised to receive the sequential diuretic treatment (Group A) and 50 to receive the combined diuretic treatment (Group B). Table 1 shows the main clinical and biochemical parameters of patients with cirrhosis divided into Group A and Group B according to randomisation. No difference was observed with respect to gender, age, aetiology of cirrhosis, duration of ascites, scores of severity of the liver disease, and common laboratory parameters between the two groups. As reported in table 2, no difference was observed in MAP, HR, renal plasma flow, glomerular filtration rate, renal sodium handling, NOx, PRA, plasma aldosterone concentration, or plasma vasopressin concentration between the two groups of patients.
Flow diagram of patient allocation to treatment and patient response to treatment. More detailed information is shown in the text.
Baseline clinical characteristics and laboratory common tests in patients randomised into sequential diuretic therapy (Group A) and combined diuretic therapy (group B)
Baseline parameters of systemic haemodynamic, renal haemodynamic and function and baseline plasma sodium concentration, plasma renin activity, plasma aldosterone concentration and nitric oxide metabolites (NOx) in patients randomised into sequential diuretic therapy (Group A) and combined diuretic therapy (group B)
As reported in fig 2, patients who reached an effective step of diuretic therapy were 44 (88%) out of 50 in Group A and 48 (96%) out of 50 patients in Group B (p = NS). In Group A, the effective diuretic step was step 1 in 13 patients (29.55%), step 2 in 18 patients (40.91%), step 3 in 11 patients (25.00%) and step 4 in two patients (4.55%). In Group B, the effective diuretic step was step 1 in 26 patients (54.71%), step 2 in 16 patients (33.33%), step 3 in six patients (12.50%) and step 4 in two patients (4.55%). The mean time for achieving the effective diuretic step was significantly shorter in Group B than in Group A (5.0 (SD 2.0) days vs 6.6 (SD 2.4) days, p<0.0025). The effective diuretic treatment had to be stopped in two patients in each group for an excessive diuretic response and in 14 patients of Group A and eight patients of Group B, respectively, for the development of adverse effects (table 3). As a result, as reported in table 3, the total number of patients who failed to respond to treatment was 22 in Group A (44%) and 12 in Group B (24%) (p<0.05). In patients who did not fail to respond to treatment (28 in Group A and 38 in Group B, p<0.05), the mean time for the resolution of ascites was shorter in Group B than in Group A (15.5 (SD 5.6) vs 20.7 (SD 6.4) days, p<0.001). No difference was observed in the loss of body weight (6.1 (SD 1.7) kg vs 5.3 (SD 2.2) kg, p = NS), or in changes in serum sodium (1.4 (SD 3.5) mEq/l vs −0.2 (SD 3.3) mEq/l, p = NS), serum potassium (−0.3 (SD 0.4) mEq/l vs −0.1 (SD 0.3) mEq/l, p = NS), serum urea (−1.3 (SD 1.1) mg/l vs −1.0 (SD 1.2) mg/dl, p = NS), and serum creatinine (0.04 (SD 0.00) mgl/dl vs 0.07 (SD 0.01) mg/dl, p = NS) between the two groups at the end of treatment.
Prevalence of treatment failures (no achievement of an effective diuretic step, excessive response or adverse effects while on effective diuretic step) in patients randomised into sequential diuretic therapy (Group A) and combined diuretic therapy (Group B)
Considering patients who developed adverse effects prior to or after having achieved the effective diuretic step as a whole (table 4) were 19 in Group A (38%) and 10 (20%) in Group B (p<0.05). The diuretic steps at which these patients dropped out from the study for the development of adverse effects are reported in table 5. Individual values of laboratory parameters in patients who developed adverse effects are reported in table 6. It should be noted that 15 out of 19 patients (71.63%) who developed adverse effects in Group A did so during monotherapy with potassium canrenoate (steps 1 and 2). Well in keeping with this observation, a higher rate of hyperkalaemia in patients of Group A was observed (table 4). Potassium canrenoate was temporarily withdrawn in all patients who developed hyperkalaemia. In addition, three patients also received a potassium chelant.
Patients who developed adverse effects either prior to or after achieving the effective diuretic step
Diuretic step at which patients dropped out from the study due to the development of adverse effects
Individual values of serum potassium, serum sodium and serum creatinine in patients who developed adverse effects prior or after the achievement of an effective diuretic step
Fourteen out of 22 patients who failed to respond to treatment in Group A could be effectively treated by combined diuretic treatment, while the other eight patients required therapeutic paracentesis. Five out of 12 patients who failed to respond to treatment in Group B were effectively treated by changing the dose of potassium canrenoate and/or furosemide, while the other seven patients were treated with paracentesis.
During the study no other complication was observed. One non-responder in each group developed a bacterial infection after exclusion from the study.
Discussion
Sequential diuretic therapy, ie, administering increasing doses of an anti-aldosteronic drug and adding a loop diuretic only in non-responders to the highest recommended dose of the anti-aldosteronic drug,2 is an effective and safe approach for the treatment of ascites in patients with cirrhosis.7 8 Nevertheless, it has often been hypothesised that combined diuretic therapy, namely, using a potassium sparing diuretic and a loop diuretic simultaneously, can offer two major advantages over the sequential diuretic therapy: (1) a greater and faster effect on urinary sodium excretion due to the synergic inhibitory effect of the two diuretics on renal sodium reabsorption at different tubular sites; and (2) less frequent development of hyperkalaemia because the loop diuretic counteracts the potassium sparing diuretic of the anti-aldosteronic drug.6 14 15 Consequently, combined diuretic treatment is recommended by the AASLD guidelines for the treatment of ascites in patients with cirrhosis5 and is used worldwide,16 17 18 despite the results of the only randomised comparative study which has been published up to now on this specific issue. In that study, Santos et al6 showed that the combined diuretic therapy has no advantage over spironolactone alone in the treatment of ascites in non-azotaemic patients with cirrhosis. Despite similar results on efficacy and safety, the combined diuretic treatment gave a higher number of patients who had an excessive response to diuretics, thereby making their management more complex.6
The results of the present study are quite different from those of Santos et al6 and they provide a substantial basis to the choice of using combined diuretic schedule in the treatment of ascites in patients with cirrhosis. First of all, the combined use of potassium canrenoate and furosemide made it easier and faster to find out an effective step of diuretic treatment. Patients who received the combined diuretic treatment (Group B) were quite similar to those who received the sequential diuretic treatment (Group A) in terms of renal haemodynamics, renal function, and overall, in terms of the delivery of sodium to the sites of action of both diuretics used in the study. Therefore, this finding can be only attributed to the faster and greater effect that the simultaneous use of potassium canrenoate and furosemide has on urinary sodium excretion. Second, combined diuretic therapy was safer than the sequential one. The number of patients who developed diuretic-induced side effects was greater in Group A than in Group B. Looking more closely at the adverse effects, what made the difference in the present study was the higher prevalence of hyperkalaemia in patients of Group A, which most frequently occurred during the phase of monotherapy with potassium canrenoate. As a consequence of its better tolerability, the combined diuretic treatment made it possible to resolve ascites in a larger number of patients without changing the effective diuretic step when compared to the sequential diuretic treatment. Finally, the simultaneous use of potassium canrenoate and furosemide reduced by almost a week the time required to achieve the resolution of ascites.
It may be difficult to explain why the results of the present study are so different from those of Santos et al.6 However, it should be underlined that Santos et al compared spironolactone alone versus the combined use of spironolactone and furosemide rather than sequential versus combined diuretic treatment. As a consequence, the most striking difference between the two studies lies in the quite different prevalence of patients who responded to the anti-aldosteronic drug alone. This prevalence was 94.00% in the study by Santos et al while it was 70.45% in the present study. Clinical and laboratory features of the patients included in the two studies should be considered in order to explain such a difference. Looking at the data by Santos et al6 it is evident that more than 50% of patients had never had ascites and had never used diuretics before inclusion into the study. In addition, they had no or at least only a moderate baseline activation of the renin–angiotensin–aldosterone system.6 In this type of patient the high efficacy of the monotherapy with an anti-aldosteronic drug is well proven by previous studies, including our previous prospective clinical trial.7 However, when patients have a history of recurrent ascites as well as a clear-cut baseline activation of the renin–angiotensin system at inclusion, as was the case in most of the patients who were included in the present study, the efficacy of the monotherapy with spironolactone or other anti-aldosteronic drugs decreases to less than 60%.7 8 Nevertheless, taking into account that the maximum effect of anti-aldosteronic drugs is reached after 4–6 days the efficacy of monotherapy with an anti-aldosteronic drug could have been greater in Group A if the period of treatment in the first two steps had been longer.
As far as the adverse effects are concerned, it should be underlined that their rate was lower than 10% in both arms in the study by Santos et al6 while it was higher in both arms in the present study, well in keeping with previous observations showing that the rate of adverse effects to diuretics ranged between 19%7 8 and 40%.19 20 21 This difference is related to the more restricted criteria that were used to define adverse effects and to the fact that a potassium chelant was not used in the present study.
In conclusion, the results of the study proved for the first time that the combined diuretic therapy is safer and quicker compared to the sequential diuretic therapy in the treatment of moderate ascites in patients with cirrhosis and without renal failure.
Acknowledgments
We are grateful to Ms M Canapero, a native English speaker at the University of Padova, for proofreading this paper.
REFERENCES
Footnotes
Funding The work was supported in part by a grant “ex 60%” from the Ministry of Scientific Research and of the University of Italy.
Competing interests None.
Ethics approval The study was approved by the ethics committee of the University and General Hospital of Padova (registration number 318P) on 9 April 2005.
Provenance and Peer review Not commissioned; externally peer reviewed.