Article Text
Abstract
Objective Stem cell transplantation provides a promising alternative for the treatment of fulminant hepatic failure (FHF). However, it lacks fundamental understanding of stem cells’ activities. Our objective was to clarify stem cell-recipient interactions for overcoming barriers to clinical application.
Design We used an in-house large-animal (pig) model of FHF rescue by human bone marrow mesenchymal stem cells (hBMSCs) and profiled the cells’ activities. The control and transplantation groups of pigs (n=15 per group) both received a D-galactosamine (D-Gal) injection (1.5 g/kg). The transplantation group received hBMSCs via intraportal vein infusion (3×106 cells/kg) immediately after D-Gal administration. The stem cell-recipient interactions were quantitatively evaluated by biochemical function, cytokine array, metabolite profiling, transcriptome sequencing and immunohistochemistry.
Results All pigs in the control group died within an average of 3.22 days, whereas 13/15 pigs in the transplantation group lived >14 days. The cytokine array and metabolite profiling analyses revealed that hBMSC transplantation suppressed D-Gal-induced life-threatening cytokine storms and stabilised FHF within 7 days, while human-derived hepatocytes constituted only ∼4.5% of the pig hepatocytes. The functional synergy analysis of the observed profile changes indicated that the implanted hBMSCs altered the pigs’ cytokine responses to damage through paracrine effects. Delta-like ligand 4 was validated to assist liver restoration in both pig and rat FHF models.
Conclusions Our results delineated an integrated model of the multifaceted interactions between stem cells and recipients, which may open a new avenue to the discovery of single molecule-based therapeutics that simulate stem cell actions.
- STEM CELLS
- FULMINANT HEPATIC FAILURE
- CYTOKINES
Statistics from Altmetric.com
Significance of this study
What is already known on this subject?
Fulminant hepatic failure (FHF) is a life-threatening condition for which liver transplantation is an effective treatment option.
Stem cell-based therapy represents a promising alternative for patients with FHF and has significant implications in clinics.
The precise mechanism of stem cell-recipient interactions after stem cells transplantation remains unclear.
What are the new findings?
Human bone marrow mesenchymal stem cell (hBMSC) transplantation rescued FHF pigs through suppressing life-threatening cytokine storms and stabilised FHF within 7 days.
The proliferation and transdifferentiation of the implanted hBMSCs replaced only ∼4.5% of the hepatocytes in recipient pig liver.
The implanted hBMSCs altered the recipients’ cytokine responses to damage through paracrine effects.
An important cytokine/biological process, Delta-like ligand 4/Notch, may participate in restoring the damaged liver in FHF.
How might it impact on clinical practice in the foreseeable future?
Mechanistic understanding of stem cell-recipient interactions might be helpful in overcoming barriers to clinical translation and developing novel single molecule-based therapeutics that simulate stem cell actions.
Introduction
Fulminant hepatic failure (FHF) is a lethal condition characterised by widespread hepatocyte necrosis, acute deterioration of liver function and subsequent multiorgan failure.1 ,2 Orthotopic liver transplantation is currently viewed as one of the best effective treatment options for FHF, but donor organ shortages, contraindications and irreversible hepatic encephalopathy result in the death of many patients awaiting liver transplantation.3 ,4 Therefore, alternative therapeutic strategies for FHF are greatly needed.5 ,6
Stem cell transplantation has shown substantial potential in treating many lethal diseases.7–9 Small-animal and preliminary human studies have indicated overall improvement in the regeneration of damaged organs.6 ,10 ,11 However, the exact mechanisms through which stem cells assist organ repair are still elusive, and the preliminary results of several nonrandomised clinical trials have demonstrated a level of efficacy insufficient for these cells’ application as a first-line therapy.11 Therefore, fundamental understanding of stem cell-recipient interactions is required for the successful development and optimisation of stem cell-based therapies.12
Stem cell application in FHF treatment is attractive, but many questions remain unanswered.13 As we and others have reported, liver repair through stem cell proliferation and transdifferentiation into hepatocytes has been postulated as the major mechanism of this therapy's action.5 ,14 Recent studies also indicated a substantial role for paracrine effects in delivering overall benefits, although no specific signalling molecule has yet been identified to mediate these paracrine effects.15 ,16 The relative importance of these two mechanisms is also a topic of controversy.12 Furthermore, we still do not know the timeframe in which stem cells act to stop disease progression. Answers to these key questions will be the foundation of designing further translational studies on the development and optimisation of stem cell-based FHF therapy.
To overcome these barriers to clinical translation, in the present study, we used our own in-house translational model, namely human bone marrow mesenchymal stem cell (hBMSC)-mediated rescue of d-galactosamine (D-Gal)-induced FHF in pigs, to delineate stem cells’ activities after transplantation. The stem cell-recipient interactions were quantitatively evaluated by biochemical function, cytokine array, metabolite profile, messenger RNA sequencing (mRNA-seq) and immunohistochemistry (IHC) (figure 1).
Scheme of human bone marrow mesenchymal stem cell (hBMSC) transplantation for rescuing fulminant hepatic failure (FHF) in pigs. To understand the fundamental interactions between implanted stem cells and recipient hosts with FHF, we quantitatively profiled stem cells’ activities after transplantation using an automated biochemistry analyser, cytokine arrays, ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS), messenger RNA sequencing (mRNA-seq) and immunohistochemistry (IHC).
Materials and methods
Isolation, culture and identification of hBMSCs
hBMSCs were isolated by bone marrow aspiration from the iliac crest of healthy male volunteers. More details on the methods of hBMSC isolation, culture, phenotypic identification and multilineage differentiation are available in online supplementary materials and methods. Cells were used for experiments during passages 3–7.
Supplemental material
FHF models
All experimental protocols were approved by the Animal Care Ethics Committee of the First Affiliated Hospital, Zhejiang University, and all animals received humane care according to the criteria of the ‘Guide for the Care and Use of Laboratory Animals’.17 The experimental methods were carried out in accordance with the approved guidelines. Briefly, male Chinese experimental miniature pigs (Taihe Biotechnology, Jiangsu, China) weighing 8–10 kg and aged 2.5 months underwent FHF induction with D-Gal at a dose of 1.5 g/kg body weight via jugular vein catheterisation. Additionally, male Sprague–Dawley rats (Zhejiang Academy of Medical Sciences, Hangzhou, China) weighing 200–250 g and aged 2 months underwent FHF induction with D-Gal at a dose of 3.0 g/kg body weight via intraperitoneal injection.14
hBMSC transplantation in pigs with FHF
Two groups (n=15/group, randomised assignment) of pigs with FHF were studied: an intraportal hBMSC transplantation (T) group, in which the animals received a transfusion with 3×106 hBMSCs/kg suspended in 10 mL of normal saline (NS) via the intrahepatic portal vein under B-ultrasound guidance immediately after D-Gal injection, and a control (C) group, in which the animals underwent a sham procedure with an equal volume of NS without cells. No animal received any medical support (eg, infusions, drugs) during the entire experimental period.
Delta-like ligand 4 treatment in both pigs and rats with FHF
Two groups (n=15/group, randomised assignment) of pigs with FHF were studied: a Delta-like ligand 4 (DLL4) treatment group, in which the animals received multiple injections of DLL4 at a dose of 0.03 µg/kg (2 mL) through the jugular vein at 12, 36, 60 and 84 h after D-Gal induction, and a control (CTL) group, in which the animals underwent a sham procedure with an equal volume of NS without DLL4. The beneficial role of DLL4 was also validated in the male SD rat model (200–250 g) with FHF. Two groups (n=50/group, randomised assignment) of rats with FHF were also studied: a DLL4 group, in which the animals received multiple injections of DLL4 at a dose of 0.2 µg/kg (1 mL) through the intraperitoneal route at 8, 14, 20, 26, 32, 38 and 44 h after D-Gal induction, and a CTL group, in which the animals underwent a sham procedure with an equal volume of NS without DLL4. No animal received any medical support (eg, infusions, drugs) during the entire experimental period.
Survival curve analyses
Animal survival was monitored for up to 2 weeks after hBMSC transplantation and DLL4 treatment in pigs and rats with FHF. Survival curves were compared using the non-parametric Mantel–Cox test. Animal survival was observed by a blinded expert with 10 years of experience in animal studies.
Materials, reagents, antibodies and PCR primers
The materials, reagents and antibodies, and quantitative reverse transcription PCR (qRT-PCR) and primers for RT-PCR used in the study are provided in online supplementary tables S1–S3, respectively.
Supplementary tables
Sample collection
Serum, peripheral blood mononuclear cells (PBMCs) and liver tissues were collected from the experimental animals according to the experimental design for a period of 14 days after FHF induction. The serum samples were used in biochemical tests, ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS) analysis, cytokine array detection and ELISA. The PBMCs were profiled via mRNA-seq. Finally, the liver tissues were profiled/analysed using UPLC-MS, H&E staining, IHC, mRNA-seq and qRT-PCR. Sequencing reads from our mRNA-seq experiments are available in the Sequence Read Archive database (accession number: SRP063325). Detailed methods are available in online supplementary materials and methods.
Statistical analysis
The results of the measurements are presented as the mean±SEM, unless otherwise noted. No statistical method was used to predetermine sample sizes. Comparisons between two groups with a sample size >3, except the comparisons of the most significant pig-derived cytokine expression, were performed using the non-parametric Mann–Whitney U test, assuming two-tailed distributions. Comparisons between two groups with a sample size of 3 and the comparisons of the most significant pig-derived cytokine expression (n=5 per group) were performed with the heteroscedastic Welch's t test to ensure sensitivity, assuming two-tailed distributions. The normality assumption of Welch's t test was validated using the Shapiro–Wilk test. The sample data in each compared group showed p>0.05. Comparison of metabolite profiles, represented by the first two principal components, was performed using the nonparametric MANOVA permutation test (the R ‘vegan’ Package). False discovery rate (FDR) calculated with the Benjamini–Hochberg procedure was used to control type I error in multiple tests. The criteria ‘p<0.05, FDR<0.25’ was used in detecting biochemical marker changes, ‘p<0.05, FDR<0.1’ in detecting serum cytokine changes and ‘p<0.05, FDR<0.05’ in detecting transcript changes. Hierarchical clustering was performed using the R ‘stats’ Package, involving the Euclidean distance matrix and the Pearson product-moment correlation coefficient.
Results
Human BMSC transplantation stabilised FHF in pigs within 7 days
Phenotypic analysis by standard flow cytometry showed that seventh-passage hBMSCs were positive for CD29 and CD90 but negative for CD34 and CD45. The results of multipotential differentiation showed that the hBMSCs could differentiate into osteocytes, adipocytes and hepatocyte-like cells. These findings indicated that the cells used in this study possessed typical hBMSC phenotypes and multipotential stem cell characteristics (see online supplementary figure S1). All pigs in the control group died within an average of 3.22 days, whereas 13/15 pigs in the transplantation group lived beyond day 7 (figure 2A), and all of these 13 pigs survived for up to 6 months. The other two pigs died within 4 days, and subsequent autopsy indicated that FHF pathology resulted in rapid death.
Human bone marrow mesenchymal stem cell (hBMSC) transplantation stabilises fulminant hepatic failure (FHF) by day 7 (D7). (A) Survival analysis. (B) Differentially regulated biochemical markers. (C) Trajectory of metabolite profiles plotted with their first two principal components (PC1, PC2). (D) Temporal changes in six typical biochemical markers of liver function. (E) Differentially regulated cytokines. (F) H&E staining of liver tissue. hBMSC transplantation led to notable improvements in the liver tissue structure at D7. C, control group; T, transplantation group. D3:D0, day 3 vs day 0; D0-D1, day 0 to day 1. Error bars, SEM. Mann–Whitney U test, **p<0.01, *p<0.05. Scale bar, 100 μm. (A) n=15/group; (B, D) n=5–15/group; (C) n=10–15/group; (E) n=3/group; (F) n=5/group.
Biochemical assay analysis showed that 21 biomarkers in the control group and 22 biomarkers in the transplantation group exhibited significant changes at day 3 relative to day 0 (figure 2B). Among these biomarkers, 20 were shared between the two groups. Transplantation attenuated the 18 of the 20 biomarker changes (see online supplementary figure S2). Cytokine array analysis indicated that hBMSC transplantation altered liver responses to damage, conferring a protective effect at day 3 (detailed later in a dedicated section). UPLC-MS profiling of the serum metabolites indicated a significant difference in hepatic metabolic functions between the two groups, which was observed as early as at day 1 (p=0.012, figure 2C). These results indicated that implanted hBMSCs produced immediate protective effects within the first three days after transplantation.
At day 7, most (24/35) of the biochemical markers were restored to their baseline levels in the transplantation group (see figure 2D for six representative markers), except two significantly downregulated biomarkers and nine significantly upregulated ones (figure 2B). Similarly, in the transplantation group, no monitored cytokine exhibited a significant difference between days 0 and 7 (figure 2E). Consistent with these results, UPLC-MS analysis (figure 2C) of 2768 distinguishable compounds in the serum revealed no significant differences between the metabolite profiles at days 0 and 7 in the transplantation group (p=0.813). In contrast, these compounds shifted to right on day 3 in the control group (p=0.001). H&E staining (figure 2F) further confirmed that transplantation was notably coupled with repair of the damaged liver structure at day 7, whereas the deceased control animals showed a typical FHF histology with extensive hepatic necrosis and haemorrhage. These results indicated that hBMSC transplantation stabilised FHF within the first seven days after D-Gal administration.
Furthermore, as shown in the online supplementary figure S2, the levels of low, very low and high density lipoprotein, and total cholesterol were significantly decreased in both groups after D-Gal administration and they restored to baseline on day 5–7 after hBMSC transplantation. These findings also indicated that the implanted hBMSCs restored hepatic synthetic functions and stabilised FHF within the first seven days.
Human-derived hepatocytes replaced approximately 4.5% of pig hepatocytes
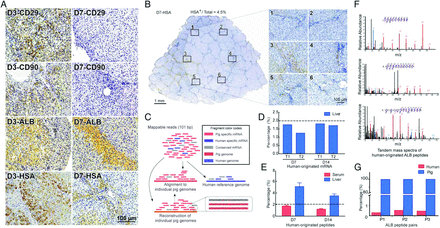
To understand the interactions between stem cells and recipient pig hosts, we quantified the relative contribution of cell replacement to the course of hBMSC-assisted liver restoration. IHC using human-specific antibodies showed that both undifferentiated hBMSCs (CD90+ and CD29+) and human-derived hepatocytes (albumin (ALB)+ and hepatocyte-specific antibody (HSA)+) were found in the pigs’ liver tissue at day 3, but only human-derived hepatocytes were found after day 7 (figure 3A, and see positive and negative controls in online supplementary figure S3). These data suggested that transdifferentiation of the implanted hBMSCs was close to completion at day 7.
Proliferation and transdifferentiation of transplanted human bone marrow mesenchymal stem cells. (A) immunohistochemistry of liver tissue from pigs in the T group. (B) Percentage (4.5%) of human-derived hepatocytes in the livers of T-group pigs at day 7 (D7). (C) Separation of human-originated and pig-originated mRNA sequencing reads. (D) Percentage of human-derived gene expression in liver tissues from two pigs (T1, T2). (E) Percentage of human-derived peptides from liver tissue and serum. (F) Typical MS/MS spectra of the human-derived albumin (ALB) peptides. (G) Percentage of human-derived ALB from liver tissue. P1, P2, P3: three pairs of peptides that distinguish between human- and pig-derived ALB. C, control group; T, transplantation group. Scale bar, 100 μm. Error bars in (D) and (E), SEM. (A, B) n=5; (D) n=2; (E) n=3, 5 at D7 and D14 for serum; n=3 both at D7 and D14 for liver tissue; (G) six samples harvested from three pigs were pooled together for analysis.
The percentage of human-derived hepatocytes in the liver tissue was estimated by counting HSA+ cells (figure 3B). Human-derived hepatocytes constituted approximately 4.5% and 4.7% of the liver hepatocytes at days 7 and 14, respectively (see online supplementary figures S4–S7). Consistent with these results, mRNA-seq analysis showed that 1.3–1.8% of the total transcripts in the liver were human derived (figure 3C, D). UPLC-MS analysis of peptides in the liver tissue and of secreted peptides in the serum indicated that 3.5–5.1% and 1.2–1.7%, respectively, were human derived (figure 3E). ALB secretion is a characteristic function of hepatocytes, and UPLC-MS analysis of three pairs of peptides that distinguish between human-originated and pig-originated ALB indicated that only 0.4% of the ALB in the liver tissue was human derived (figure 3F, G). Therefore, proliferation and transdifferentiation of the implanted hBMSCs replaced ∼4.5% of liver hepatocytes when FHF was stabilised. However, the percentage of liver function compensated for by transplantation-derived cells could be lower, as evidenced by the lower levels of human-derived gene expression and ALB production in the liver tissue.
hBMSCs altered liver responses to damage through paracrine effects
To explore the paracrine interactions between hBMSCs and recipient pig hosts, we measured the changes in the serum levels of 215 cytokines using cytokine arrays (see online supplementary table S4), and randomly selected six of significantly changed cytokines for validation using ELISA (see online supplementary figure S8). In the control group at day 3 relative to day 0 and in the transplantation group at days 3, 7 and 14 relative to day 0, cytokine changes were clustered (figure 4A). The D-Gal-induced life-threatening cytokine storms, observed in both groups, were suppressed in the transplantation group at day 7. In the transplantation group, in particular, 46 cytokines showed significant changes at one or more time points (see online supplementary table S5). Hierarchical clustering analysis revealed four co-regulated cytokines clusters (see online supplementary figure S9). Most (36/46) of the cytokines exhibited a change pattern with a peak at day 3 that returned to a near-baseline level at day 7 and remained stable at day 14 (see online supplementary figure S9A, cluster 1). This pattern aligned with the progression of FHF and its restoration after hBMSCs transplantation. Other (10/46) cytokines showed alternative temporal change patterns (see online supplementary figure S9A, clusters 2–4). Functionally, these cytokines were related to inflammation, regeneration, apoptosis, immunoregulation or metabolism (see online supplementary figure S9B).
Paracrine effects of transplanted human bone marrow mesenchymal stem cells. (A) Heat map of 215 protein-level changes relative to day 0 (D0). (B) Venn diagram of the differentially regulated cytokines from D0 to D3 in the control (C) and transplantation (T) groups (p<0.05). (C) Heat maps of differentially regulated cytokines involved in the most significantly altered functional themes: immunoregulation (top), development (left bottom) and apoptosis (right bottom). See online supplementary table S4 for full cytokine names. (D) Quantitative reverse transcription PCR (qRT-PCR) measurement of human- and pig-derived EPCAM (EpCAM, Epithelial cell adhesion molecule), KDR (vascular endothelial growth factor receptor 2 (VEGF-R2)) and ENG (endoglin) in the liver tissues. Only pig-derived mRNAs were detected at D3 in T group. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal reference. The results are representative of five independent experiments. H, human; P, pig; HN, human normal liver tissue; PN, pig normal liver tissue; T-D3, liver tissue harvested from the T group at D3. Error bars, SEM. Student's t test, *p<0.05. D3:D0, D7:D0, D14:D0, day 3 or day 7 or day 14 vs day 0. (A–C) n=3/group; (D) n=5/group.
In the cytokine storms at day 3, 57 cytokines showed significant changes in either one or both groups (figure 4B). Surprisingly, specific changes observed in only one group (49 changes) were more frequent than common changes observed in both groups (8 changes), suggesting a disparity in the liver responses to damage between the two groups. From a functional perspective, most (48/57) of the altered cytokines had functions related to signal transduction, metabolism, apoptosis/cell death, immunoregulation or development (see online supplementary table S5). Cytokines from many pleiotropic signal transduction pathways and many metabolic processes were differentially induced between the two groups, indicating a fundamental difference in the regulation of cell behaviours (see online supplementary figure S9C,D). Interestingly, the altered cytokines in the control group were more frequently involved in the regulation of apoptosis and cell death (5/27 changes in the control group vs 1/38 changes in the transplantation group). By contrast, the altered cytokines in the transplantation group were more enriched with functions in immunoregulation (1/27 changes in the control group vs 12/38 changes in the transplantation group) and developmental control (2/27 changes in the control group vs 5/38 changes in the transplantation group) (figure 4C, and annotation enrichment analysis results in online supplementary table S6). Comparison of liver gene expression between pigs with transplantation (at day 7) and pigs without (at day 3) confirmed this trend of response divergence (see online supplementary table S7).
The qRT-PCR using human-specific mRNA further confirmed that the pigs’ cells produced the three most significant changes in the serum cytokine levels that were specifically observed in the transplantation group (changes in EpCAM, VEGF-R2 and endoglin levels; figure 4D). These results indicated that the implanted hBMSCs remodelled the pigs’ liver responses to damage by releasing small amounts of regulatory cytokines.
Profiling analysis indicated DLL4-Notch activation
To identify the important signalling processes through which the implanted hBMSCs exerted their paracrine effects, we analysed the gene expression changes in PBMCs. PBMCs responding to soluble factors without the complication of the tissue microenvironment may exhibit signalling changes that are more directly related to the paracrine effects of implanted hBMSCs. We also identified the differentially expressed genes in both the control and transplantation groups, and differentially co-expressed genes between the two groups (see online supplementary table S8). Differential co-expression is defined as a cluster of genes that show highly correlated expression in one group (evidence of physiologically relevant activation of a biological process), but not in another group. Functional synergy analysis of expression changes showed 23 altered signalling processes (table 1) including several well-known signalling processes (TLR,18 tumour necrosis factor-alpha, interleukin (IL)-1 and IL-619) that respond to liver damage. Two cytokines regulating these signalling processes (stem cell factor and IL-6) have been reported to exert prophylactic effects against FHF.20 ,21 The rediscovery of these signalling processes suggested the potential to exploit other identified processes in FHF treatment.
Twenty-three generalised biological process annotations analysed by gene set linkage analysis in peripheral blood mononuclear cells
Of the identified 23 signalling processes, the Notch signalling pathway is of particularly interest. The differentially expressed genes in the control and the transplantation groups and the differentially co-expressed genes between the two groups all have frequent functional interactions with the Notch signalling pathway (figure 5A). Notch receptors have five known ligands;22 we compared the expression of these ligands in the liver tissue (the transplantation group at day 7 vs the control group at day 3) and found that in the transplantation group, the expression of DLL1 was suppressed (p=0.026) and that the expression of DLL4 was induced (p=0.004, figure 5B). Validation using qRT-PCR and ELISA confirmed that DLL4 was specifically induced in the transplantation group at both the mRNA and the protein levels (p<0.05, figure 5C,D).
Delta-like ligand 4 (DLL4)-Notch activation in human bone marrow mesenchymal stem cell (hBMSC)-directed liver restoration. (A) Summary of evidence linking DLL4-Notch signalling to liver restoration. DE, differentially expressed genes; D3:D0, D7:D0, day 3 or day 7 vs day 0; T-DcoE, D0-7:D0-3, differentially co-expressed gene clusters, which were co-expressed in the transplantation (T) group between D0 and D7 but which showed no correlation in the control (C) group between D0 and D3. (B) Differences in the expression levels of Notch-related ligands in the liver tissue between the T group at day 7 (D7) after d-galactosamine administration and the C group at D3. (C) Quantitative reverse transcription PCR (qRT-PCR) measurement of DLL4 expression in liver tissue. *p<0.05, Mann–Whitney U test. (D) ELISA measurement of serum DLL4 protein. *p<0.05, Student's t test. (E) Survival analysis of the DLL4-treated pigs/rats (p=0.033/0.045, Mantel–Cox test). CTL, control group. (F) H&E staining of liver tissue. DLL4 treatment led to notable improvements in the liver tissue structure at D7. Scale bar, 50 μm. Error bars in (C), SEM. (B) n=2/group; (C) n=5/group; (D) n=3/group; (E) n=15/group (pigs), n=50/group (rats); (F) n=5/group.
Survival benefit of DLL4 in pig and rat FHF models
DLL4 treatment notably improved the survival of both pigs and rats with FHF (p<0.05, figure 5E). In the validation using the pig model, the pigs in the control group had a mean survival time of 2.6 days, whereas the pigs in the DLL4 treatment group survived for 4.6 days on average (two pigs survived for up to 6 months). Similarly, in the validation using the rat model, the rats survived for 5.3 days on average in the control group and for 7.1 days in the DLL4 treatment group. No animal received additional medical support (eg, nutrient infusions, drugs) during the entire experiment. H&E staining indicated that the liver structure was notably repaired after DLL4 treatment (figure 5F). These results demonstrated that DLL4 treatment alone improves animal survival.
Discussion
Patients with FHF with life-threatening cytokine storms and deficiencies in liver detoxification and metabolic and immune regulation may rapidly die within several weeks.2 ,4 Stem cell transplantation holds substantial promise for the clinical treatment of FHF.6 To overcome the barriers to clinical application, many questions (such as questions about the optimal timing, transdifferentiation and paracrine effects of stem cell therapies) need to be answered.13 ,23 In the present study, we quantitatively delineated stem cell-recipient interactions via multifaceted profiling and demonstrated that implanted stem cells rescue D-Gal-induced FHF by suppressing cytokine storms and alter the recipient's (here, the pig's) response to liver damage. Importantly, we discovered that DLL4, as a potential therapeutic molecule, may participate in the rescue of FHF via hBMSC transplantation (figure 6).
Proposed mechanisms of human bone marrow mesenchymal stem cell (hBMSC) action in liver restoration. d-galactosamine (D-Gal) injection destroys hepatocytes, inducing the release of detrimental cytokines, which further damage the liver tissue. Implanted hBMSCs respond to damage signals and proliferate and transdifferentiate to repair the liver tissue. Through paracrine effects, the hBMSCs also induce the expression of cytokines in the host; these cytokines are involved in immunoregulation and development, which protect liver cells and programme the liver responses to damage. We found evidence of an important role for Delta-like ligand 4 (DLL4)-Notch activation in the hBMSC-directed liver restoration programme.
Understanding the action time of implanted stem cells is useful guidance to ensure optimal timing of treatment in future clinical applications.24 Certain evidence in cardiomyocyte transplantation research has suggested the existence of a temporal window of opportunity bound by the acute inflammatory response, on the one hand, and by scar formation, on the other.25 However, the experimental and clinical data on the action timing after stem cell transplantation are limited. In the current study, our in-house large-animal model of FHF rescue demonstrated that immediate transplantation of hBMSCs after D-Gal administration prevented death from FHF in the initial three days. Serum metabolite profiles and biochemical changes indicated an immediate protective effect of the implanted hBMSCs by suppressing D-Gal-induced life-threatening cytokine storms. This timeframe provides evidence supporting direct investigation of the timing of stem cell-based therapy in a clinical setting.
How stem cells act, and specifically whether cell replacement mechanisms or the cells’ paracrine effects contribute most to stem cell-assisted organ repair, remains a topic of controversy.12 Liver repair through stem cell proliferation and transdifferentiation has been postulated as the major mechanism of stem cell action in treating FHF.5 ,14 Several studies showed that only 1–12%26–28 of hepatocyte mass was replaced in the recipient liver; such estimates were typically generated by counting immunohistochemically stained cells or by flow cytometry. To obtain a more reliable estimate, here, we used IHC, mRNA-seq and UPLC-MS to quantitatively evaluate the number of human-derived hepatocytes at two time points. Our results show that at day 7, when FHF was stabilised and differentiation completed, implanted hBMSCs replaced ∼4.5% of the pig hepatocytes. However, the liver function compensated for by these cells could be even lower (0.4%–1.8%), as indicated by the lower levels of human-derived gene expression and ALB production. The fraction of liver cells replaced by differentiated hBMSCs seems too low to explain the significantly improved animal survival, which suggests the importance of paracrine effects in hBMSC-assisted liver restoration.
The pivotal role of paracrine effects in stem cell therapies has been recognised to contribute to many biological processes, such as by preventing inflammation, inhibiting apoptosis, improving metabolism and promoting regeneration.15 ,12 Certain positive results based on paracrine mechanisms have been reported in ischaemic stroke, myocardial infarction and kidney injury. 7 ,12 ,29 In the present study, the importance of paracrine effects was further supported by the observation of immediate protective effects within the first three days after transplantation, when hBMSC differentiation was far from complete. Furthermore, the paracrine effects of the implanted hBMSCs altered the recipient response to damage, from producing more apoptosis/cell death-related cytokines to producing more cytokines related to immunoregulation and developmental control, but the most altered cytokines were not produced by transplantation-derived cells. These results indicated a high-level regulatory role for the stem cells’ paracrine effects and suggested potential for the discovery of stem cell-inspired molecular therapies via understanding the components of stem cells’ secretome and their functions. However, many metabolic processes specifically induced by the implanted hBMSC need to be further clarified.
Although recent studies indicated that systemic infusion of BMSC-conditioned medium improved survival in a small-animal (rat) model of FHF,16 ,30 certain components may be potentially harmful.31 ,32 Searching for key therapeutic molecules that may be useful for developing a balanced cocktail, instead of whole cells, is emerging as an exciting concept in regenerative medicine. However, no single molecule-based treatment for disease had been developed based on stem cell actions prior to the current study.15 Because the beneficial factors are mostly high-level regulatory factors present in small quantities, in profiling analysis, changes in these factors are easily masked by changes in downstream factors and effector proteins that exist in large quantities. In this study, we used a novel procedure that cross-references the functional changes in biological processes against the expression changes in their known regulators to discover small beneficial regulatory changes and discovered that differential expression of DLL4 in the transplantation group was beneficial for liver restoration. We observed that a characteristic feature of the transplantation effects was the induction of cytokines that direct development. DLL4-Notch activation might be one important step underlying the hBMSC-directed liver restoration programme. Recent studies have indicated that DLL4, along with other Notch ligands, contributes to biliary injury repair.33 Moreover, our validation results demonstrated that DLL4 treatment at 8–12 h post-D-Gal administration significantly improved survival in both pig and rat models of FHF. These results are useful for further designing a novel single-molecule-based therapy by simulating stem cell actions for future clinical applications.
In summary, our quantitative evaluations delineated an integrated model of the multifaceted interactions between stem cells and their recipients. Furthermore, we discovered that DLL4, as a potential therapeutic molecule, might be useful for developing a novel therapy that simulates stem cell actions for the treatment of FHF. However, the precise mechanisms of DLL4 expression and how it works to improve liver function need to be further clarified, and for future therapy to be clinically effective, fine-tuning of the intervention with regard to timing, dosing, the route of administration and long-term follow-up also needs to be performed.
Acknowledgments
The authors thank Dr Guoqiang Mo for giving helpful suggestions in animal experiments and Dr Yu Chen for providing animal administration.
References
Footnotes
Contributors DS, JZ, QZ, JX and JJ contributed equally. The study was designed by JL and supervised by JL, XC and LL. The manuscript was written by JL, XC, ML, JZ and DS. The experiment and data analysis were performed by DS, JZ, QZ, JX, JJ, LJ, TW, JL, WD, JL, SS, JL, NZ, LZ, LJ, SH, PC, HC, ML, LL, XC, and JL. All authors were involved in critical revision of manuscript.
Funding This work was supported by the National Basic Research Program of China (2012CB944900), the Zhejiang Provincial and National Natural Science Foundation of China (LR13H030001, LR13C020001, 81271708, 81571818 and 31571356), the Chinese High Tech Research and Development (863) Program (2013AA020102), the National S&T Major Project (2012ZX10004503-006 and 2012ZX10002004-001), and the Research Fund for the Doctoral Program of Higher Education (20130101110008).
Competing interests None declared.
Patient consent Obtained.
Ethics approval The Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University.
Provenance and peer review Not commissioned; externally peer reviewed.