Article Text
Abstract
Hepatocellular carcinoma (HCC) ranks number three among the most frequent causes of death from solid tumors worldwide. With obesity and fatty liver diseases as risk factors on the rise, HCC represents an ever increasing challenge. While there is still no curative treatment for most patients numerous novel drugs have been proposed, but most ultimately failed in phase III trials. This manuscript targets therapeutic advances and most burning issues. Expert key point summaries and urgent research agenda are provided regarding risk factors, including microbiota, need for prognostic and predictive biomarkers and the equivocal role of liver biopsy. Therapeutic topics highlighted are locoregional techniques, combination therapies and the potential of immunotherapy. Finally the manuscript provides a critical evaluation of novel targets and strategies for personalized treatment of HCC.
- hepatocellular carcinoma
- clinical trials
Statistics from Altmetric.com
Introduction
The incidence of hepatocellular carcinoma (HCC), the most common primary malignancy of the liver, has risen in recent decades.1 However, new therapy research for HCC has generated a graveyard of negative trials.2–4
Gut brought together internationally recognised experts from clinical and research backgrounds to discuss progress, problems and pitfalls in HCC research.
Topics included
Risk factors and potential screening options.
Prognosis, staging and biomarkers.
Locoregional therapies and combination therapy.
Learning from failed trials.
Personalised treatments and immunotherapy.
NASH, diabetes and obesity
HCC arises in most cases in a context of chronic hepatic inflammation, and its biggest risk factor is cirrhosis.5 The causes for the underlying liver disease are diverse and comprise chronic viral infection with hepatitis B, C or D viruses, toxicity of alcohol, autoimmune and cholestatic liver diseases and metabolic factors.1 6–8
The prevalence of metabolic risk factors (obesity, type 2 diabetes or combined hypertension/dyslipidaemia/previous cardiovascular event) in patients with HCC is rising and increased significantly from 2000 to 2010.9 Non-alcoholic steatohepatitis (NASH) is now present in around 20% of incident diagnoses of HCC and this trend will continue, whereas HCV infection is present in around 50% of cases and the incidence of HCV-induced HCC is falling. Diabetes and obesity are the strongest metabolic factors associated with HCC.10
In a 2015 case–control study, having diabetes or having been obese in early adulthood increased the OR of HCC up to six times.11 The OR for HCV or HBV is 30–40, so the relative risk is considerably smaller. However, prevalence of diabetes and obesity is much larger and rising. Moreover, obesity is often associated with habits and lifestyles which can also increase the risk of HCC.12
One unresolved question is the relative importance regarding HCC of alcohol consumption in patients with a metabolic syndrome. This is a frequent clinical situation which has been so far neglected.
Several publications suggested that a significant proportion of patients with HCC and metabolic syndrome present without cirrhosis13–15 so their cancers are less likely to be diagnosed by screening, as patients enrolled in surveillance programme are cirrhotics. This stresses the need to develop screening tests to identify HCC in non-cirrhotic individuals with metabolic syndrome, diabetes and obesity. While a single nucleotide polymorphism in the PNPLA3 gene has been associated with HCC in patients with NASH, its role in a surveillance programme remains to be defined.16
In comparison to patients with HCC due to other causes, patients with NASH and HCC are likely to be older and have more comorbidities, which is associated with a worse prognosis. Because they often do not have cirrhosis, they are more likely to be resected—but outcomes may be affected by steatosis, which may jeopardise the regenerative capacity of the liver and lead to more surgical site infections (figure 1).
Steatosis and surgical site infections. (SSI) BMI, body mass index.
The choice of antidiabetic therapy may affect the risk of developing HCC. Insulin treatment has been reported to increase the risk of HCC (OR 4.37), while metformin seems to reduce HCC risk (OR 0.79).17
Key points and questions
The prevalence of metabolic risk factors, especially diabetes and obesity, has risen among patients with HCC.
Screening tests to identify HCC among patients who are non-cirrhotic with metabolic risk factors are needed.
How important is alcohol consumption in patients with metabolic syndrome, with regard to disease progression and HCC?
The gut microbiome
While there has been much interest in the possibilities of predicting or even treating HCC risk by adjusting the microbiota, most research is at a preclinical stage.
The composition of the gut microbiota is largely stable in health, with differences mainly at species level. Studies in humans found that the microbiota differ in people with NASH. Enterobacteria and Proteobacteria are found in increased numbers, whereas anti-inflammatory bacterial strains such as Faecalibacterium prausnitzii are decreased, suggesting bacterial imbalance, that is, dysbiosis.18–23
Similar findings emerge from studies of the microbiota of individuals with liver cirrhosis.24 Bacterial diversity is decreased in patients with liver cirrhosis. Proinflammatory strains including Veilonella (more usually found in the oral cavity) seem to be increased in the gut of these patients. The gut microbiota has so far not been well studied in HCC patients.
All studies of microbiota and HCC are therefore preclinical. In mouse models of laboratory-induced liver cancers, gut microbes influenced the development of tumours, inducing tumourigenesis.25 A study which investigated metabolites produced via the action of microbiota on food found that a certain prebiotic (an inulin-type fructan) reduced the proliferation of liver cancer cells in mice, perhaps by stimulating the production of the short-chain fatty acid propionate.26
Hepatocarcinogenesis after toxic liver injury has been shown to depend on the intestinal microbiota and TLR4.27 In this report, treatment with antibiotics was able to suppress tumour formation especially in later stages of hepatocarcinogenesis.
Treatment with a probiotic mixture (Lactobacillus rhamnosus GG, Escherichia coli Nissle, VSL#3) was able in a mouse model to reduce subcutaneous HCC growth accompanied by reduction of IL-17 and other angiogenesis factors.28 There exists, however, an urgent need for human data in HCC on the role of the intestinal microbiota and for experimental interventional studies.
At present, the field is insufficiently advanced to use microbiome assays to screen for HCC and none of the microbiota studies have so far shown a direct effect on hepatocarcinogenesis.
Key points and questions
Liver cirrhosis is associated with profound gut dysbiosis.
Microbiota research in HCC is at a preclinical stage.
Animal studies have found a potential role for probiotics in the prevention of hepatocarcinogenesis.
Is HCC development in humans affected by intestinal microbiota?
Staging and prognosis
Patients need and expect information about their disease stage and prognosis, to help them share decisions about treatment. They need to know their expected outcome with and without treatment, the risk of recurrence after treatment, and the risk of death after treatment.
Staging linked to first-line treatment indication can help clinicians guide patients through this decision-making process.
Researchers also need reliable ways to stage disease and predict prognosis. Without accurate characterisation of patients in cohort studies, populations cannot be well targeted. Badly-targeted study populations lead to unexpected results. Good staging and prognostic guidelines are also needed to calculate study sample size, to estimate an expected survival size in uncontrolled early studies and to stratify patients to ensure balance between arms of a randomised controlled trial (RCT).
The most widely used strategy is the Barcelona Clinic Liver Cancer (BCLC) Staging and Treatment Strategy.3 Other systems such as the Hong Kong Liver Cancer strategy have been proposed in order to allow more patients to undergo therapy with intention to cure.29 Nevertheless, this strategy has not been validated and the goal of any system should not be only to apply more interventional therapies, but rather extend survival.
The authors indicate that the Child-Pugh and MELD classifications, cited in their model, are not able to identify all end-stage patients with cirrhosis. Factors such as variceal bleeding, malnutrition, hepatorenal syndrome and arterial hypotension reflect more advanced liver failure and as such affect prognosis. Patients with liver cancer do not progress through the evolutionary stages of the disease in a linear fashion and are more complex than existing classifications allow.
Surrogate markers of survival, usually disease control or time to progression (TTP), are often used in early trials. Progression under treatment is used in oncology to transition across sequential lines of therapy, and this is the main use. However, surrogate markers do not always translate into better overall survival.30 Obviously, the absence of progression is beneficial in patients with cancer, but not all progressions have the same impact on prognosis and there is no proof that TTP is a surrogate of survival in patients with HCC.
An analysis of trials of sorafenib versus placebo found no correlation between TTP and survival.31 What is important is rather the pattern of progression during treatment with sorafenib32
Growth of intrahepatic or new intrahepatic sites.
Growth of extrahepatic sites.
New extrahepatic sites or new vascular invasion, as recently validated within the regorafenib trial.33
Analysis of survival data based on these patterns shows a marked difference in prognosis, from best to worst. Growth of intrahepatic lesions may have no dismal impact on a patient’s prognosis and indeed, progression of any type may not reflect treatment failure.
Failure to take account of the pattern of progression under therapies known to be effective may induce a flaw in second-line trials, as an imbalance in the profile between trial arms may prime the trial for a misleading result.
Key points and lessons
Patients and researchers need reliable ways to stage disease and predict prognosis.
Surrogate markers of survival such as TTP do not always translate to overall survival.
Studies should take more account of the pattern of progression under therapy.
Biomarkers
Early HCC diagnosis needs to be improved, and the identification of subgroups of HCC patients with different prognosis and response to treatment would be highly valuable for their clinical management.
Two established histoprognostic factors are tumour differentiation and vascular invasion. However, single tumours can show differing degrees of differentiation, and vascular invasion can usually only be assessed on surgical samples. These drawbacks limit the performance of standard tumour biopsy and strongly support the need for surrogate biomarkers.
Molecular subtypes are one area of interest. Several gene signatures have been published, which fall within two major subgroups34–36
HCC proliferation class, which shows a more aggressive phenotype.
A non-proliferation class linked to better prognosis.
However, although gene signatures have now been published, they are not used in practice. Reasons limiting use include:
Mandatory tumour biopsy is not advocated in clinical guidelines.
Gene signatures are complex and their use limited. In addition, most gene signatures have been obtained during retrospective investigations from surgical tumour samples, so it is unclear how relevant they are to in-situ biopsy samples.
Potential sampling variability of biopsy give the tumour heterogeneity.37 38
A recent study with prospective design has failed to validate the predictive power of any of the proposed signatures.39
Gene signatures, once identified, can be translated to protein markers,40 which in some cases have been shown to have prognostic value at either early or late stage disease. Intratumour genetic heterogeneity has been detected in most HCC cases,38 and may reflect tumour aggressiveness. Molecular heterogeneity can make predictions by a biopsy not fully accurate.
To biopsy or not to biopsy?
The question of whether clinicians should take more biopsies of the tumour and surrounding liver provoked debate.41
HCC is usually diagnosed and treated without a biopsy, based on radiological findings. Some argue that if the diagnosis is already established by imaging, clinical decision-making will not be affected by biopsy results (either positive or negative). If imaging is not specific, a biopsy is mandatory to make the diagnosis. However, if the diagnosis is in place and the result of a biopsy would not change management, it would be unethical to impose a biopsy outside of a research protocol.
Others say that the field of HCC will not advance while hepatologists resist taking biopsies, and that individual patients may benefit from additional information about their prognosis.
In other oncological fields, genetic markers derived from analysis of tumour biopsies have allowed clinicians to fine-tune therapies. In some cancer areas, only a small minority of patients benefit from a specific therapy, but genetic markers allow the identification of those patients. Biopsies taken with ethical approval in clinical trials might advance this identification in HCC.
There may be subgroups of patients in negative HCC therapy trials who gained benefit because their tumour had a genetic susceptibility to the treatment. However, without the genetic markers to identify those subgroups, the overall failure of the trial means no patients benefit.
New approaches may help. Tumour heterogeneity can be identified by in-situ approaches such as matrix-assisted laser desorption/ionisation (MALDI) mass spectrometry, which ‘paints’ tissue with lasers.42 It has been used with some success to identify subpopulations in breast and gastric cancer.43
Another new method is liquid biopsy, a non-invasive procedure that detects circulating tumour cells and nucleic acids from plasma.44 45 The presence of circulating tumour cells has been associated with poor overall survival, increased risk of disease recurrence and death.46 Nevertheless, these findings need to be validated and false positives require explanation.
The way forward for better stratification of patients with HCC may be a combination of markers from radiology and pathology, as well as clinical markers. Currently, there are no validated, useful biomarkers, and the lack of biopsy material means this is unlikely to change without change in practice or breakthrough in techniques that do not require a tissue biopsy. Another option is to develop research with prospective biopsy sampling.
Key points and questions
There is a need for reliable, reproducible biomarkers to improve early diagnosis and subgroup detection in HCC.
Molecular signatures have been identified but validation has not been achieved.
New approaches including MALDI and liquid biopsies are under development.
Should clinicians routinely take biopsies from tumour and surrounding tissue outside of clinical trials?
Locoregional therapies
Two main types of locoregional therapy are in current use, ablation and embolisation. Each has its place within the BCLC strategy.
Ablation can be curative and has been used in place of resection as a first-line therapy. However, when compared with resection, ablation showed worse overall survival in one of three RCTs.47
Further analysis of trial data suggests that ablation is most successful in small tumours (<3–4 cm in diameter).
The most commonly used therapy is radiofrequency ablation (RFA). Other techniques include microwave ablation (MA), cryoablation and irreversible electroporation ablation (IRE) and are becoming more commonly applied. MA may improve ablation of larger tumours. Heat damage is not an issue with cryoablation and IRE, but more clinical data are required to establish their place in therapy.
Embolisation includes a wide variety of techniques. Transarterial chemoembolisation (TACE) is widely used, with an established survival advantage over best supportive care for non-resectable cancer.48 In TACE, the tumour is injected with a chemotherapeutic drug (often suspended in lipiodol) and the blood supply is interrupted to increase dwell time and minimise washout.
Alternatives include embolisation, without the use of a chemotherapy drug (TAE), embolisation with drug-eluting beads (DEB-TACE) and radioembolisation (Y90).
TAE has supporters as a simple and reproducible therapy with promising survival data. However, no comment can be made on survival benefit when compared with best supportive care.49
DEB-TACE has not shown a survival advantage compared with TACE using lipiodol. However, patients with more advanced disease may tolerate better and benefit more than those with less advanced disease, in whom there is no advantage over TACE.50 51
Some studies of DEB-TACE have shown impressive survival outcomes in the region of 47 months in selected patients.51 However, this degree of success depends on very careful patient selection.
Several studies are ongoing into radioembolisation, which is attractive partly because it is simple, reproducible and can be delivered as an outpatient treatment. It can be used to treat a lobe, segment or the entire liver, as required. However, no survival benefit has been demonstrated compared with conventional TACE.
Radioembolisation has been used as a ‘bridge’ to resection, for patients with cirrhosis who might otherwise have been considered unresectable.
One small trial comparing Y90 with DEB-TACE showed no difference in overall survival, TPP or progression-free survival. However, patients treated with Y90 had an average 1.5 treatments, whereas patients treated with DEB-TACE had an average 3.8 treatments.52
One recent randomised trial comparing Y90 with TACE showed significantly better TTP with Y90 (>26 months) than TACE (6.8 months).53
While TTP continues to be a challenging outcome, it is nonetheless recommended as the primary endpoint in randomised phase II studies.
Assessing an overall survival benefit in early and intermediate HCC attributable to the initial treatment is challenging. Analysis of overall survival in intermediate and advanced HCC can be complex, because of the difficulty in accounting for therapy crossover. Patients who progress on the starting therapy in a trial are changed to another, and perhaps then to a third-line therapy. Hence, it becomes difficult to attribute overall survival benefit to the initial therapy.
Recently, the results of two prospective randomised trials, SARAH54 and SIRVENIB,55 aimed at demonstrating survival superiority of Y90 over sorafenib failed to meet their primary endpoints. Further insight into the data will be forthcoming as the publications become available.
Key points and questions
Ablation is most successful for small tumours (<3 cm) and RFA is most commonly used.
Embolisation by TACE shows survival advantage for non-resectable HCC.
Radioembolisation is an alternative but has not shown survival benefit over TACE.
How do we perform overall survival studies in intermediate HCC?
Combination therapies
The record of combined therapies in HCC has been mixed. The biggest trial of combination therapy in HCC—resection or RFA with or without adjuvant sorafenib—was a surprise failure, showing no improved survival or TTP.56
A meta-analysis of three studies of RFA with or without TACE, all conducted in Asia, was more positive.57 But detailed analysis shows overall survival and recurrence-free survival were only significantly improved in people with tumours >3 cm, reflecting the already-good performance of RFA on small tumours.
The most success has been shown in use of locoregional therapy before liver transplant. While it does not improve outcomes, TACE is a good predictor of post-transplant outcome. Where TACE reduces tumour size by 50%, patients have a better chance of disease-free survival post liver transplant—71% at 5 years, compared with 49% in the group not selected by TACE.58 Rather than seeing TACE and liver transplant as adjuvants, TACE can be considered a selection tool for transplant.
Combination therapy with TACE and sorafenib in the recent SPACE study proved no better than TACE alone, measured either by TTP or overall survival,59 and these findings have been confirmed in a more recent study.60
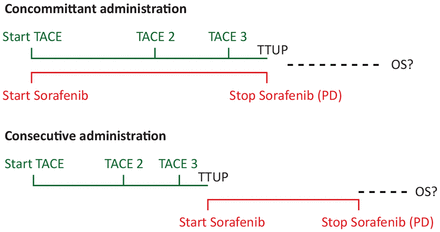
While the concept of combining an intervention known to be effective with a drug known to be effective sounds promising, studies of resection, RFA and TACE combined with sorafenib have been unsuccessful. This calls for reconsideration of the concept (figure 2).
TACE and sorafenib: combine, but how? OS, overall survival; PD, progressive disease; TTUP, time to untreatable progression.
The aim when combining an intervention with a drug is to prolong the TTP to the next intervention, until a stage is reached where no further intervention is possible. The alternative strategy is to use the primary intervention alone, then the second, until no further intervention is possible—at which point you would begin drug treatment.
There is no evidence that concomitant administration delivers better overall survival than consecutive administration. However, side effects and quality of life for the patient may well be better with sequential treatment.
Combination drug therapy raises similar questions. There is no evidence to date that using any two drugs concurrently works better than using a single drug. However, sequential use of sorafenib followed by regorafenib does improve survival.33 61
The striking finding here is the length of survival overall, which is in the region 26–29 months when measured from initiation of the first drug (sorafenib).62 This may be attributable to hyperselection of patients, but the results show impressive progress. At the preclinical level there are also evidences suggesting the efficacy of combining sorafenib with several compounds, such as recently the selective CDK4/6 inhibitor palbociclib.63
The findings also call into question the expected life expectancy with standard therapy alone. In some trials, survival in the control group (TACE) is lower than expected, making results in the combination arm hard to read. Median survival of <20 months implies a deviation from the current standard recommendations and should be in the region of 24 months when following EASL and AASLD 2011 guidelines.
The requirement to show improved survival over a study where the control arm is expected to survive 30 months presents difficulties, which is a reason why trial investigators use TTP as a surrogate marker for success. However, this may not be informative.
Key points and questions
The use of therapies in combination has had mixed success, but combined RFA/resection plus sorafenib, and TACE plus sorafenib have not shown improved survival.
These failures suggest reconsideration of the concept of combination therapy.
Is sequential use of therapies better than concomitant use of therapies in combination?
Why do promising compounds fail?
The litany of failed phase III trials for compounds to treat HCC is lengthy. Unsuccessful trials of compounds and drug combinations for HCC include
Sunitinib64
Linifanib65
Combined sorafenib and erlotinib66
Combined sorafenib and doxorubicin67
Everolimus70
Ramucirumab71
Arginine depletion72
Tivantinib73
Trials failed on one or more of these:
Efficacy of compound being tested
Unacceptable toxicity levels
Inappropriate trial design, especially imbalance of prognostically relevant factors in the different trial arms.
We can learn from successful trials how to maximise the chances of success. Very effective drugs are less dependent on good trial design, because their efficacy will be obvious. However, good design and patient selection can identify efficacy in compounds suitable for some patient groups.
Part of the problem in identifying an effective compound is how to identify a truly promising signal in phase II trials.
If we look at the phase II trial of sorafenib, treatment response and radiological progression did not suggest efficacy.74 Yet when the researchers allowed treatment until symptomatic progression in their phase III endpoints, it gave the studies time to generate positive overall survival results.61 75
The other key point from the early sorafenib trials was the understanding that liver function and performance status influence the patient’s prognosis as well as tumour burden.3 76 This suggests the need for careful selection and stratification of patients in RCTs to ensure balance between treatment and control arms.
Toxicity can be a major problem when testing compounds to treat HCC. In the RESORCE trial of regorafenib, the patient population comprised patients who had already tolerated sorafenib, a similar drug, raising the likelihood that they would tolerate regorafenib. This trial design also specified a high level of patient stratification.33
The phase III trial of lenvatinib is based on impressive phase II trials in patients with advanced unresectable HCC.77 Very recently, at American Society of Clinical Oncology (ASCO), the results of the phase III were presented, demonstrating lenvatinib non-inferior to sorafenib in overall survival in a first-line setting for unresectable HCC.78
A recently published pooled exploratory analysis of the SHARP and Asia-Pacific phase III studies, in which sorafenib significantly prolonged overall survival, showed that aetiology is not relevant for prognosis, as the geographic difference is due to a more advanced stage at recruitment in Asia. Moreover, significantly increased sorafenib benefit on overall survival was found in patients without extrahepatic spread of the neoplastic disease, and in those with HCV infection, factors that should be taken into account in the analysis of trials.79
Key points and lessons
Patient selection is key for studies, so that antitumour efficacy can translate into improved survival. Patients need good liver function and performance status.
Toxicity is crucial and phase II trials have not been sufficiently robust to give sufficient information on this limiting factor.
TTP in phase II trials and treatment response are insufficiently helpful markers for success. Symptomatic progression, progression pattern and overall survival should be considered.
Consideration should be given to conducting phase II RCTs, and these trials should use stratification to ensure properly balanced treatment arms.
‘All-comer’ trials might become no longer appropriate. The future may lie in treating subgroups of patients defined by the molecular biology and genetic profile of the tumour and the surrounding tissue (figure 3).
Molecular-based trial design in HCC. Recent trials aimed at including clinically uniform patients. Future approaches should acknowledge molecular information to identify functionally relevant alterations which can be targeted in specifically enriched populations characterised by molecular uniformity. HCC, hepatocellular carcinoma.
Immuno-oncology
One area of great interest in all fields of cancer is immuno-oncology.80 This relatively new field may generate therapies appropriate for HCC because it is an inflammation-induced cancer, (for instance)81 82, and spontaneous immune response is often seen. Immune cells correlate with outcome in HCC, and T-cell infiltration of a tumour is prognostic of a better outcome.83
Immunology treatment can be independent of liver function, because metabolism is not involved, and it combines well with the ablative therapies already used in early HCC. The concept is to activate T cells with ablation and boost their antitumour action, using a variety of mechanisms including checkpoint blockade and blockade of immunosuppressive cytokines.
How does the theory translate into practice? A small proof-of-concept study in 30 HCC patients treated with RFA and a monoclonal antibody checkpoint inhibitor, tremelimumab, showed positive initial results in some patients.84 Patients with HCV and HBV showed improvement in both tumour and underlying viral disease. Biopsies taken before and during treatment showed therapy leads to infiltration of CD8 T cells in the tumours of responsive patients (figure 4).
HCC patients were treated with anti-CTLA4 plus ablation. Shown are tumour responses over time in form of a spider blot. Only responses in non- ablated lesions were measured.
Three early stage HCC immunotherapy trials have been reported thus far; two using tremelimumab (anti-CTLA4) and one using nivolumab (anti-PD1), another checkpoint inhibitor.84–86 The use of cytokine-activated killer cells has also been investigated, with some success.87 At the more experimental stage, there is huge interest in CAR T cells, using glypican-3 or alpha-fetoprotein (AFP) as an agent. Other experimental research is ongoing into oncolytic viruses and vaccines.
Because checkpoint inhibitors have been successful in other indications, they are now leading the field. However, there is interest in other ways to target T cells and in other targets. The liver offers a great variety of targets, including T cells, immunogenic cell death, enhanced antigen presenting cells (APC) function, macrophages, cytokines and chemokines. Molecular signatures may help identify patients responding to immunotherapy as recently suggested.88
The initial role for immunotherapy in HCC may be an alternative to sorafenib in patients who cannot tolerate the drug. Immunotherapies could potentially be added to RFA or resection, TACE or sorafenib, and compared with sorafenib and regorafenib. However, these potential uses are all subject to ongoing or future research in order to prove a survival benefit.
Key points and questions
Immunotherapy can be carried out independently of liver function.
Early studies have shown some success in a range of immunological approaches.
Should immunological therapies be combined with locoregional therapies and/or also with sorafenib?
New targets and strategies for personalised treatments
Next-generation sequencing of tumours has deeply modified our understanding of the cancer genome. In HCC, whole-exome sequencing shows 40–60 somatic coding mutations per tumour, but most are passengers that are stochastic mutations without functional consequences. Sequencing suggests each tumour has about four to six driver mutations—functional mutations that target the key signalling pathway involved in liver carcinogenesis89–91 (figure 5).
The multistep process of carcinogenesis on cirrhosis. The role of TERT promoter mutations in the early step of carcinogenesis and the accumulation of genetic alterations during tumour progression are represented. Genes in blue are tumour suppressor genes and in red oncogene. HGDN, high grade dysplastic nodule; LGDN, low-grade dysplastic nodule; RMN, regenerative macronodule.
Six areas of the genetic landscape may be relevant for targeting in HCC34
Telomere maintenance. The TERT promoter mutation is the most frequent somatic genetic alteration in HCC and these mutations are very early events in tumourigenesis occurring in premalignant nodules on cirrhosis.92 93 However, there is no strong telomerase inhibitor available for use.
Cell cycle gene and the P53 pathway with mutations of TP53, RB1 and CDKN2A.
Oxidative stress pathway with mutations of KEAP1 and NFE2L2.
The Wnt/beta-catenin pathway with mutations of CTNNB1 and AXIN1.
Epigenetic modifiers with mutations of ARID1A and ARID2.
The AKT/mTOR and MAP kinase pathway with mutations of TSC1/2 and RPS6KA3.
Moreover, recurrent amplifications of VEGFA and FGF19 have been described in <10% of the HCC.
Unfortunately, the most frequently identified genetic alterations found in HCC (TERT promoter 50%–60%, TP53 20%–40%, CTNNB1 15%–40%, ARID1A mutations 10%–20%) are not associated with currently available targeted therapies.89 94 However, it has been previously estimated that 28% of patients have at least one damaging alteration that is potentially targetable by an FDA-approved drug and 86% have an alteration potentially targetable by a drug currently in clinical trials in human cancer.89 For example, FGF19 amplification and overexpression could be targeted using FGFR4 inhibitors95 96 and this combination is currently tested to treat HCC in humans.
The difficulty is knowing the consequences of each mutation at a cellular level in order to differentiate passenger from driver mutations, to identify good mutation targets. Only then is it worth looking for a drug that may target the mutation. The absence of driver mutation linked with a specific targeted therapy will invariably lead to failure of biomarker-guided trial in HCC.
A salutary example is the failure of tivantinib. Tivantinib was thought to be a MET inhibitor, and was trialled for advanced HCC with MET overexpression, after a successful phase II trial in this subgroup.97 However, a phase III trial showed no difference in overall survival.73
Overexpression of MET at the protein level in HCC is common, but this is rarely linked to a DNA alteration. MET overexpression is not per se responsible for an oncogene addiction and may simply identify a highly proliferative tumour. In addition, tivantinib may not actually suppress the MET pathway in HCC cell lines and should not be considered as a MET inhibitor.98 This raises the question of whether the negative results of the tivantinib trial should be extrapolated to real MET inhibitors.
Consequently, tumour sequencing is a mandatory step to allow understanding of the disease, development of new therapies and biomarkers that could predict response or resistance to systemic treatment. To achieve this goal, tumour biopsy and biobanking should be mandatory in clinical trials. Ideally, this tissue sampling should be done prior to trial entry.
Key points and questions
Gene sequencing has identified six areas relevant for targeting HCC.
Tumour sequencing is needed to improve understanding of disease.
Negative results of tivantinib should not be extrapolated to other MET inhibitor therapies.
How to better characterise the consequences of mutations to identify targets for therapy ?
What is the impact of tumour heterogeneity and how to deal with it ?
How to develop new targeted therapies directed against the main genetic alterations of HCC ?
Conclusion
The failure of phase III trials in HCC, the difficulties in establishing biomarkers and genetic targets linked to drug therapies, all point towards insufficient understanding of the basic biology of HCC.
Successful drugs need strong biological targets, and good understanding of the mode of action of the drug. More public funding is likely to be required for a better understanding of carcinogenic mechanisms. Similarly, further investment is needed in preclinical studies to allow more solid evaluation of drug efficacy and mode of action in relevant animal models (ie, immunocompetent mice, presence of steatosis, fibrosis and cirrhosis).
In addition, the field needs more robust and careful trial design, with careful consideration of what constitutes a useful signal from a phase II trial, before progressing to phase III. This may include expansion of outcomes to include symptomatic progression, rather than censoring patients at the point of radiological progression; overall survival from an early stage; and pattern of progression as a predictor or overall survival. Surrogates of overall survival as endpoints are needed.
The place of biopsy in both clinical and research fields remains controversial. While many feel strongly that the field of HCC will not progress without routine use and analysis of biopsy tissue, this raises ethical questions, especially for patients being treated outside of clinical trials.
Many options are opening in HCC therapy, from combination immunotherapy with locoregional therapy to the possibilities of personalised treatment. But if these possibilities are to translate into improved outcomes for patients, the field needs to be underpinned by more solid basic understanding of the disease.
Acknowledgments
Anna Sayburn facilitated the meeting and provided medical writing support.
Matias A Avila is part of the Hepacare Fundación La Caixa Research Project.
References
Footnotes
Contributors All authors were involved in drafting and reviewing the manuscript and had approval of the final version submitted.
Competing interests J-FD Advisory committees: Abbvie, Bayer, BMS, Falk, Genfit, Gilead Science, Intercept, Lilly, Merck, Novartis. Speaking and teaching: Abbvie, Bayer, BMS, Genfit, Gilead Science, Novartis. Unrestricted research grant: Bayer. JB Consultancy for Abbvie, Arqule, Bayer, BMS, Boehringer Ingelheim BTG, Eisai, Gilead, Kowa, Novartis, Onxeo, Roche, Sirtex and Terumo. Research contract with Bayer. MP-R Investigator: Abbott, Arqle-Daiichi, Bayer, BMS, Boehringer-Ingelheim, Gilead, Imclone, Novartis, Roche. Speaker, Advisor: Abbott, Bayer, BMS, Boehringer-Ingelheim, Gilead, MSD, Roche. Grant Support: Abbott, Bayer, Gilead, MSD, Roche. DSMB: Lilly-Imclone, ONXEO. RS Advisor to Bayer, BTG, Terumo, Merit, Cook. PRG Honoraria for lecturing and participation in advisory boards from Bayer, Lilly, Sirtex, Sillajen, BMS, MSD.
Provenance and peer review Not commissioned; externally peer reviewed.