Article Text
Abstract
Objective Gut microbiota metabolises bile acids (BA). As dysbiosis has been reported in inflammatory bowel diseases (IBD), we aim to investigate the impact of IBD-associated dysbiosis on BA metabolism and its influence on the epithelial cell inflammation response.
Design Faecal and serum BA rates, expressed as a proportion of total BA, were assessed by high-performance liquid chromatography tandem mass spectrometry in colonic IBD patients (42) and healthy subjects (29). The faecal microbiota composition was assessed by quantitative real-time PCR. Using BA profiles and microbiota composition, cluster formation between groups was generated by ranking models. The faecal BA profiles in germ-free and conventional mice were compared. Direct enzymatic activities of BA biotransformation were measured in faeces. The impact of BA on the inflammatory response was investigated in vitro using Caco-2 cells stimulated by IL-1β.
Results IBD-associated dysbiosis was characterised by a decrease in the ratio between Faecalibacterium prausntizii and Escherichia coli. Faecal-conjugated BA rates were significantly higher in active IBD, whereas, secondary BA rates were significantly lower. Interestingly, active IBD patients exhibited higher levels of faecal 3-OH-sulphated BA. The deconjugation, transformation and desulphation activities of the microbiota were impaired in IBD patients. In vitro, secondary BA exerted anti-inflammatory effects, but sulphation of secondary BAs abolished their anti-inflammatory properties.
Conclusions Impaired microbiota enzymatic activity observed in IBD-associated dysbiosis leads to modifications in the luminal BA pool composition. Altered BA transformation in the gut lumen can erase the anti-inflammatory effects of some BA species on gut epithelial cells and could participate in the chronic inflammation loop of IBD.
- Inflammatory Bowel Disease
- Bile Acid
- Intestinal Microbiology
- Inflammatory Mechanisms
Statistics from Altmetric.com
Significance of this study
What is already known on this subject
-
Microbiota impacts on gut homeostasis.
-
Dysbiosis is involved in inflammatory bowel diseases (IBD) pathophysiology.
-
Bile acids (BAs) metabolism depends of gut microbiota activity.
-
BAs exerts anti-inflammatory effects on macrophages.
What are the new findings
-
Fecal dysmetabolism of BAs is observed in IBD.
-
This dysmetabolism is linked to IBD-associated dysbiosis.
-
High rates of sulphated BAs is found in faeces of IBD patients.
-
Dysmetabolism of BAs could impact on inflammatory loop in IBD.
How might it impact on clinical practice in the foreseeable future?
-
BAs dysmetabolism could be used as a surrogate marker of IBD.
-
Modulation of gut microbiota and/or BAs content could impact on IBD clinical course.
Introduction
Ulcerative colitis (UC) and Crohn's disease (CD), the two main inflammatory bowel diseases (IBD), are characterised by chronic and relapsing inflammation of the gastrointestinal tract.1 Although their pathogenesis remains puzzling, it is thought to involve the inappropriate and ongoing activation of the mucosal immune system driven by the presence of intestinal microbiota.2 ,3 The human gut microbiota represents the highest cell densities (1013–1014 micro-organisms)4 recorded in any ecosystem. Two phyla, Bacteroidetes and Firmicutes, dominate gut microbiota biodiversity.5 The gut microbiota exerts physiological functions useful for the human host such that human metabolism is the result of both microbial and human attributes.4 However, deviation away from gut microbial balance, or ‘dysbiosis’, has been repeatedly reported in IBD and may have an impact on host metabolism.6 ,7
Convergent data in IBD point to dysbiosis characterised by restricted biodiversity, temporal instability8 and decreased bacteria of the Firmicutes phylum.9 Among Firmicutes, Faecalibacterium prausnitzii appears to be particularly under-represented in IBD patients.6 ,10–12 Within the gut lumen, enzymatic reactions performed by gut bacteria are responsible for bile acid (BA) biotransformation. BA is synthesised by the liver into its primary forms, namely cholic acid (CA) and chenodeoxycholic acid (CDCA), and then conjugated with glycine or taurine amino acids before excretion via biliary ducts. After the secretion of BA into the gut lumen during digestion, the microbiota performs two enzymatic reactions. Deconjugation of taurine or glycine by BA hydrolase13 is the preliminary step before hydroxysteroid dehydrogenation (transformation), which leads to the production of secondary BAs.14 Active reabsorption of conjugated BAs in the terminal ileum by specific transporters permits the return of BAs to the liver via portal circulation. In the liver, free BA is reconjugated with taurine or glycine before secretion into the biliary tract and intestinal lumen. This metabolic loop constitutes the enterohepatic cycle of BA.15 Thus, IBD-associated dysbiosis could induce BA dysmetabolism and affect gut homeostasis.
In addition to the well-known metabolic functions of lipid absorption and cholesterol homeostasis, BAs also act as signalling molecules to regulate their own biosynthesis,16 and participate in gut mucosal defences through their own antibacterial properties.17 ,18 This suggests that BA dysmetabolism has the potential to greatly influence the course and/or phenotype of IBD. Indeed, BAs have been repeatedly shown to be anti-inflammatory molecules able to decrease the synthesis of proinflammatory cytokines, like TNF-α in monocytes and macrophages, through NF-κB inhibition.19–22
For this reason, we hypothesised that IBD-associated dysbiosis could lead to BA dysmetabolism, and consequently, gut inflammation. In this study, we investigated patients with purely colonic IBD and observed IBD-associated dysbiosis and an imbalance in BA profiles in both faeces and sera. These results support the idea that BA dysmetabolism in IBD is related to the loss of gut microbiota function associated with dysbiosis. In addition, experiments in vitro suggest that this imbalance in BAs may play a role in chronic inflammation in IBD.
Methods
Patients and samples
Blood (5 ml) and faecal samples were collected from 42 IBD patients (12 CD, 30 UC) during flare-ups (n=23) and remissions (n=19). A diagnosis of IBD was defined by clinical, radiological, endoscopic and histological criteria.23 All IBD patients had purely colonic diseases with no ileal involvement, according to the Montreal classification system.24 CD activity was defined as a Harvey–Bradshaw index score ≥4,25 and active UC was defined as a Powell–Tuck index score >4.26 Patients with ileal involvement, or with biliary or liver comorbidities, were excluded from the study.
None of the study participants had taken antibiotics or used colon-cleansing products for at least 3 months prior to enrolment. The characteristics of the patients are presented in table 1. Fasting blood samples were centrifuged (400×g, 10 min, 4°C) and the serum was stored at −80°C for further analysis. Whole stools were collected in sterile boxes and immediately homogenised, and 0.2 g aliquots was frozen at −80°C for further analysis. Control blood and faecal samples were obtained from 29 healthy subjects (HS) using the same protocol. Informed consent was obtained for all participants, and the study was approved by the local ethics committee (25 January 2005).
Characteristics of the study subjects
Bile-acid profiles
Chemicals and reagents
All chemicals and solvents were of the highest purity available. CA, deoxycholic acid (DCA), CDCA, ursodeoxycholic acid (UDCA), lithocholic acid (LCA), hyocholic acid and the corresponding glyco- and tauro-derivatives were obtained from Sigma-Aldrich (Saint-Quentin-Fallavier, France). The 3-sulphate derivatives were a generous gift from Dr J Goto (Niigata University of Pharmacy and Applied Life Sciences, Niigata, Japan) and the 23-nor-5 β-cholanoic acid-3α, 12α-diol was purchased from Steraloids, Inc (Newport, Rhode Island, USA). Because 3-sulpholithocholic acid is not commercially available, it was synthesised in our laboratory (ENS, Paris) using a previously described method,27 and further characterised by nuclear magnetic resonance and mass spectrometry. Acetic acid, ammonium carbonate and ammonium acetate were also purchased from Sigma-Aldrich (Saint-Quentin-Fallavier).
Standard solutions
Standard stock solutions were prepared in methanol at a concentration of 1 mg/ml and stored in a sealed container at −20°C. The stock solutions were pooled and diluted to obtain mixed-calibration BA solutions, ranging from 31.3 µg/ml to 31.3 ng/ml.
Sample preparation
Two microlitres of an internal standard solution (23-nor-5 β-cholanoic acid-3α, 12α-diol at 1 mg/ml) was added to either serum (500 µl) or 0.1 g of faecal lyophilised samples using a Thermo Savant Speedvac (SPD 111V) coupled to a cooled vapour trap (RTV400). The BAs were released from the binding protein by the addition of 0.4 M ammonium carbonate, at a concentration of 4 ml ammonium carbonate per 1 ml of sample, and incubated for 30 min at 60°C. For faecal samples, 2 ml of NaOH (0.1 M) was added and incubated for 1 h at 60°C before the addition of 4 ml of water.28 The solution was homogenised by two 30 s runs in an Ultra-Turrax disperser (IMLAB, Lille, France). The preanalysis cleanup procedure was achieved by centrifugation (20 000×g for 20 min) followed by solid-phase extraction using reversed-phase silica cartridges. These reversed-phase Chromabond C18 cartridges (100 mg; Macherey-Nagel, Düren, Germany) were preconditioned with 5 ml of methanol and 5 ml of water in succession. Samples were then loaded on the cartridge, and the subsequent elution steps were processed using a vacuum manifold. The cartridge was rinsed with water (20 ml), followed by hexane (10 ml), and then rinsed a second time with water (20 ml). The BAs were finally eluted and collected by methanol eluate. The methanol was evaporated under nitrogen at 50°C, and the residue was resuspended in 150 μl of methanol, 5 µl of which was injected into the high-performance liquid chromatography tandem mass spectrometry (HPLC MS/MS) system.
HPLC MS/MS analysis
For the HPLC MS/MS analysis, the separation of BAs as a function of polarity was accomplished using an analytical column (Pinnacle II C18, Restek, Lisses, France; 250 mm×3.2 mm (L×ID), 5 µm silica particle (Restek)) fitted on an HPLC binary pump (Agilent 1100, Agilent Technologies France, Massy, France). The transfer line from the autosampler (Agilent) and column was maintained at 35°C. The 0.3–0.5 ml/min flow rate was increased during the elution protocol. The mobile phase was composed of a mixture of ammonium acetate (15 mM, pH 5.3) and methanol. The HPLC was coupled in series with the turbo ion-spray source of the tandem mass spectrometer (QTRAP 2000, Applied Biosystems/MDS SCIEX, Concord, Ontario, Canada). Electrospray ionisation was performed in the negative mode, with nitrogen as the nebuliser gas. The temperature of the evaporation gas was set at 400°C. The ion-spray, declustering and entrance potentials were set at −4500 V, −60 V and −10 V, respectively. Collision-induced dissociation was achieved in a Q2 collision cell under various voltage potentials, depending on conjugation, and MS/MS detection was operated with unit/unit resolution in the multiple-reaction-monitoring (MRM) mode. The dwell time of the ion trap was set at 70 ms for each transition. Data were acquired using Analyst V.1.4.2 software.
MRM at low collision energy focuses on transition reactions from precursor ions to product ions after the cleavage of taurine, glycine and sulphate fragments. For glycine-conjugated BAs, m/z 432, 448 and 464 were selected as the precursor ions, and m/z 74 was selected as the product ion. For taurine-conjugated BAs, m/z 482, 498 and 514 were selected as the precursor ions, and m/z 80 was selected as the product ion. For sulpho-conjuguated BAs, the HSO4 sulphuric anion (m/z 97) from the sulphate moiety was selected as the product ion. For unconjugated BAs, m/z 375, 391 and 407 were selected as both the precursor and product ions, as no fragmentation could be identified at the low collision energy used. In addition, m/z 377 was selected for the internal standard (23-nor-5 β-cholanoic acid-3α, 12α-diol). The BA quantitation was expressed as the percentage of each specific BA (±SEM) out of the total BAs after calibration of the method, with weighed mixtures and normalisation relative to the internal standard (nordeoxycholic acid).
Bacterial analysis of faecal samples
DNA was extracted from 200 mg of faeces9 and real-time qPCR was performed as previously described.12 Briefly, a qPCR thermocycler (Stratagene Mx3000P, La Jolla, California, USA) was used to quantify the total bacteria and the dominant bacterial groups in the microbiota using a 96-well plate format with SYBR Green PCR Master Mix (2X). Each reaction was performed in duplicate in a final volume of 25 µl, with a 0.2-µM final concentration of each primer, and 10 µl of the appropriate dilutions of DNA samples. Amplifications were performed at 95°C for 10 min to denature the DNA and activate the AmpliTaq Gold Polymerase, followed by 40 cycles at 95°C for 30 s and at 60°C for 1 min. A dissociation step was added, and the dissociation curves were analysed to confirm the identity and fidelity of the amplification of SYBR Green products. The real-time species-specific 16S rRNA-targeted primers used in this study were purchased from MWG Biotech AG (Ebersberg, Germany). The primer sequences used have been described elsewhere.12 To take into account the difference in water content between faecal samples, the data for each faecal sample were normalised.29 The level observed for each particular dominant and subdominant bacterial population was subtracted from the all-bacteria content, and the results are expressed as the log 10 of the number of bacteria per gram of stool.
Caco-2 cell experiments
The human colon cancer cell line Caco-2, from the European Collection of Cell Cultures (Wiltshire, UK), was cultured in 12- or 24-well culture plates in DMEM (Invitrogen SARL, Cergy Pontoise, France), supplemented with 20% heat-inactivated foetal calf serum (FCS) and 1% non-essential amino acids (Invitrogen), at 37°C in a 10% carbon dioxide/air atmosphere. The culture media were changed every day. Experiments were initiated on days 20 and 21 after seeding (previously shown to be an early stage of differentiation, corresponding to the upper crypt lower villus stage of differentiation). The culture medium was then changed to DMEM plus 10% FCS 12 h before the BA challenge. At t0, the cells were supplemented with interleukin (IL)-1β and several BA species (CA, CDCA, DCA, LCA and LCA-3S) at increasing concentrations (from 100 µM to 500 µM). After 6 h of incubation, supernatants were removed for the IL-8 assay (inflammatory response), with extraction for HPLC MS/MS BA identification. The cells were washed and scraped into a lysis buffer (phosphate buffer saline plus 1% Triton X-100) for the protein assay, and IL-8 was delivered to the total cell-protein content. An ELISA assay was performed (DuoSet, R&D Systems, Minneapolis, Minnesota, USA). All experiments were performed in duplicate.
Measurement of BA biotransformation activity by the faecal microbiota
Ten milligrams of lyophilised stool was rehydrated in 200 µl of 4°C water before homogenisation.30 ,31 Pure BA (LCA-3S, GUDCA, California, USA) solutions were added separately for a final concentration of 5 mM and mixed with each stool homogenate at 37°C. For each sample, experiments were halted at different time points (0 min, 30 min, 180 min and 12 h) using three steps: (1) addition of 100 µl of 0.2 N NaOH, (2) shaking and (3) addition of 1 ml of 4°C water. After centrifugation (10 000 RPM for 20 min), LCA, UDCA and DCA were measured from the LCA-3S, GUDCA and CA-supplemented stool homogenate, respectively. BA extraction was performed following the proper protocol. Direct enzymatic activities were detected in each experiment by the increase in LCA, UDCA and DCA. BA concentrations were estimated by the area under curve (AUC) of specific HPLC MS/MS BA profiles and reported as the mean BA in each experiment. The results are expressed in per cent BA.
Germ-free and conventional mice
Germ-free and gnotobiotic 10- to 12-week-old male mice (C3H/HeN) were obtained from the germ-free rodent-breeding facilities of the MICALIS unit at INRA (Jouy-en-Josas, France). Throughout the study, the animals were kept inside flexible film isolators (Getinge-La Calhène, Vendôme, France) in standard macrolon cages (five mice/cage) with sterile wood shavings as bedding. The animals were given free access to autoclaved tap water and a standard pellet diet (R03-40, Scientific Animal Food and Engineering, Augy, France), sterilised by gamma irradiation at 45 kGy (IBA Meridis, Fleurus, Belgium). The isolators were maintained under controlled conditions of light (0730h–1930 h), temperature (20°C–22°C) and humidity (45%–55%). The C3H/HeN germ-free mice received 0.5 ml of a 1/100 dilution of faeces from conventional mice for 2 days by gastric gavage. After 3 weeks (time to bacterial stability), their faecal samples were studied. All procedures were performed in accordance with the European guidelines for the care and use of laboratory animals.
Statistical analysis
Statistical analyses for significant differences were performed with the Student t test for unpaired data, and by Wilcoxon's non-parametric test when appropriate. Qualitative data were compared within groups using the χ2 test. Discriminations of disease phenotype (ie, active IBD, IBD in remission and HS) based on faecal microbiota composition, and faecal BA profiles were performed. The class probability function was used. Scoring functions among five learning schemes were trained and tested from a classification task for maximising AUC. We used 31 runs of 7-fold cross-validation to test the accuracy and AUC. The ranking capability was finally assessed by the AUC. The attributes of subsets were evaluated using each learning scheme. For a feature subset selection, we used a classic iterative search-based technique to find low-dimensional projections of the data that have high AUC scores. All calculations were performed using Weka 3-7-5 open source software (http://www.cs.waikato.ac.nz/~ml/weka/index.html) from Waikato University.
Results
Dysbiosis in IBD
The faecal microbiota composition was available for all of the subjects, and is presented in table 2.29 ,32 In IBD patients in remission, dysbiosis was characterised by a decrease in bacteria from the Firmicutes phylum (ie, significant decrease in Clostridium coccoides, Clostridium leptum and F prausnitzii at a species level). In flare, dysbiosis was characterised by a more profound decrease in bacteria from the Firmicutes phylum, and an increase in Lactobacillus and enterobacteria (E coli at a species level). However, there were no significant differences between CD and UC patients both in flare and in remission (table 2).
Microbial composition in IBD patients and healthy subjects (HS)
Using the ratio between F prausnitzii and E coli as a marker of dysbiosis, a decrease in dysbiosis was observed in IBD patients, and the decrease was more significant in active IBD (Figure 1).
the ratio between Faecalibacterium prausntizii and Escherichia coli in patients with inflammatory bowel diseases (in remission and active disease) versus healthy subjects. Values are expressed as a ratio±SEM; *p<0.0001. All-bacteria results obtained by qPCR are expressed as mean of the log10 value. The ratio is calculated as the log number of Faecalibacterium prausnitzii minus the log number of E coli.
Dysmetabolism of BA in the faeces of IBD patients
Total faecal BA concentrations were similar between IBD and HS (see online supplementary file S1). The proportion of conjugated BAs increased in IBD patients compared with HS, as shown in figure 2D. The proportion of secondary BAs decreased in IBD patients, particularly during flare, as represented in figure 2B. Furthermore, a much higher proportion of 3-OH-sulphated BA was found in the faeces of patients with active IBD compared with patients with IBD in remission and HS (figure 2F). Finally, we also looked at BA concentrations in sera and did not observe any difference between IBD and HS except for secondary BA (figure 2A). Indeed, the association of normal total BA concentrations with a decrease in the secondary BA level in the sera of IBD patients suggests that this decrease is a consequence of an impaired luminal bacterial BA metabolism. Levels of primary and total BAs in the sera were in the normal range, confirming the absence of cholestasis in the study subjects. Altogether, these results suggest that IBD patients exhibit defective BA intestinal metabolism.
Proportion of faecal and sera bile acid (BA) in healthy subjects and in patients with inflammatory bowel diseases (in remission and active disease): secondary BA in sera (A) and faeces (B); conjugated BA in sera (C) and in faeces (D); sulphated BA in sera (E) and in faeces (F). Values are expressed as %±SEM of total BA; *p=0.03, **p=0.02, ***p = 0.0004.
As BA metabolism in the gut is largely the result of microbiota enzymatic activity, we accounted for both BA and microbiota profiles in a class probability function three-dimensional plot. We were able to discriminate HS from active IBD patients and IBD patients in remission (figure 3). The model description (tests and performance) of the each attribute dataset is given in supplementary file S2.
Discrimination of disease phenotype based on faecal microbiota composition and faecal bile acid profiles. Healthy subjects: white dots; Active inflammatory bowel diseases (IBD): black dots; IBD in remission: grey dots. Class probability function was used. Scoring functions were trained and tested from a classification task towards maximising AUC (see S1).
Dysbiosis leads to BA dysmetabolism
We explored a direct connection between dysbiosis and BA dysmetabolism. We chose an extreme animal condition to confirm the major role of the gut microbiota in shaping the faecal BA profile. Thus, we compared the faecal BA profile in germ-free and in conventional mice. The former exhibited virtually undetectable secondary BAs (1.8±0.2% relative to the total BA) compared with the latter mice (88±1.7%; p=0.049). Furthermore, the germ-free mice exhibited a higher proportion of conjugated BAs (86.8±0.8% vs 2.5±0.2%, respectively; p=0.049) and of 3-OH-sulphated BA (11.7±0.9% vs 1.8±0.3%, respectively; p=0.049), than did the conventional mice. These results highlight the role of the microbiota in deconjugation and dehydroxylation, and in BA desulphation (figure 4).
Bile-acid composition in the faeces of germ-free versus conventional mice according to HPLC MS/MS (E). Values are expressed as %±SEM of total faecal bile acid; *p=0.05.
Finally, we assessed the BA biotransformation directly induced by the gut microbiota of IBD patients and HS. As expected, the gut microbiota of HS was able to efficiently deconjugate, transform and desulphate BAs. However, these metabolic functions were impaired in the gut microbiota of IBD patients, particularly during flare (figure 5).
Bile acids deconjugation (A), transformation (B) and desulphation (C) induced by the gut microbiota of active inflammatory bowel diseases (IBD), IBD in remission and healthy subjects. Values are expressed as arbitrary unit±SEM.
Functional impact of BA dysmetabolism
To explore the potential consequences of BA dysmetabolism on the epithelial inflammatory response, we used a Caco-2 intestinal epithelial cell model. The effects of several types of BAs were tested on IL-1β-induced IL-8 secretion. Primary BAs (CA and CDCA) had no effect, whereas, secondary BAs (DCA and LCA)-inhibited IL-1β-induced IL-8 secretion. A dose-response relationship was observed with LCA, with no IL-8 secretion at a concentration of 500 µM of LCA. Moreover, sulphation of LCA abolished this anti-inflammatory effect (figure 6). We checked that the inhibitory effect was not related to cellular toxicity by performing an Lactate Dehydrogenase (LDH) assay, presented in supplementary file S3. Taken together, these results suggest that BA dysmetabolism in IBD could be involved in the enhancement of the epithelial inflammatory response.
Interleukin (IL)-8 secretion by Caco-2 cells after IL-1β stimulation, as measured by ELISA: (A) experiments with primary (cholic acid and chenodeoxycholic acid) and secondary (deoxycholic acid and lithocholic acid (LCA)) bile acids: and (B) experiments with sulphated and non-sulphated LCA. Values represent IL-8/total protein (pg/ml) quantities in media after 6 h.
Discussion
Here, we investigated the connections between gut microbiota and BA metabolism in IBD. We demonstrated that IBD-associated dysbiosis leads to impaired BA metabolism characterised by defective deconjugation, transformation and desulphation. Moreover, we demonstrated that IBD-associated BA dysmetabolism within the gut lumen might enhance the intestinal epithelial inflammatory response, and thus, worsen IBD.
We first assessed faecal and blood BA profiles in IBD patients and healthy controls. Although patients were not gender matched, there was no significant difference between the IBD and healthy control groups (p>0.2). To create a homogeneous group of patients, the inclusion criteria were stringent. We excluded patients with abnormal liver tests or known liver diseases to avoid basal perturbations of BA biosynthesis, which may be observed—for example, in cholestatic diseases, such as primary sclerosing cholangitis (often associated with IBD).33 ,34 As the terminal ileum is the site of BA reabsorption,35–37 CD patients with ileal involvement were also excluded by principle. A systematic search for associated BA malabsorption would have been interesting, but was impossible because neither scintigraphic nor blood diagnostic tests were available in France. Our patients were well-phenotyped patients, selected from a worldwide known database (MICISTA Saint-Antoine Hospital, Paris, France). However, we could not rule out the possibility that passive BA diffusion in the colon led to a change in the BA profile in colonic IBD patients. To preserve the integrity of the gut microbiota, all patients included had to be free of antibiotics 3 months prior to sampling.38 ,39 Thus, having cautiously enrolled IBD patients and HS, we used state-of-the-art mass spectrometry (HPLC MS/MS) to measure molecular species of BAs from faecal content. After examining these data and the faecal microbiota composition, we generated a hypothesis connecting IBD-associated dysbiosis and BA metabolism.
We then performed several experiments supporting a direct connection between gut microbiota and BA biotransformation. Deconjugation and dehydroxylation of BAs depend on gut microbiota activity. A decrease in bacteria-bearing Bile Salt Hydrolase activities might be involved in the increase in the conjugated BAs remaining in the faeces. Although one cannot rule out the role of the global decrease of gut microbiota, our results clearly indicate that the decrease in total bacteria is mainly due to a lack in Firmicutes. Among all the bacteria of the gut, Firmicutes and Bacteroidetes are the most potent deconjugating bacteria.40 ,41 Thus, a decrease in non-conjugated and secondary BAs may be considered a reliable marker of IBD-associated dysbiosis. Although enzymatic redundancy is found with BA deconjugation, measurement of the metabolic activity of faecal samples from IBD patients demonstrated impaired function, especially during flare. In vivo BA transformation depends on many bacterial strains forming a metabolic chain,42 and BA deconjugation represents a limiting step in the BA biotransformation pathway.13 ,43 Germ-free mice experiments highlighted the loss of function observed in IBD patients demonstrating increases in primary, conjugated and sulphated BA.
The present protocol was adapted for faecal samples, and HPLC MS/MS44 ,45 was able to discriminate species with high resolution, and to quantify 3-OH-sulphated BA. In sera, there was no difference in the 3-OH-sulphated BA concentration between patients and controls. This might be due to the rapid urinary clearance of sulphated BA. However, given that the gut lumen is a source of 3-OH-sulphated BA,46 ,47 these results suggest a balance between intestinal-wall BA sulphation on one hand, and microbiota desulphation on the other. Looking at our results at the level of BA species, we found primary as well as secondary sulphated BAs. A change in the BA profile with high rates of primary BAs cannot explain the excess of sulphation. To address this question, we performed a sulphatase activity assay that revealed decreased activity in the faeces of IBD patients. The high proportion of faecal 3-OH-sulphated BAs in germ-free mice supports the idea of 3-OH-sulphated BA genesis in the intestinal wall, with intestinal expression of BA sulpho-transferases in human intestinal cells.46 ,48 Other alterations due to derivative sulphation can be evoked in vivo. It is well known that gut bacteria, such as Bacteroides and Clostridium, support sulphatase activity, at least at the genomic level.49 ,50 Sulphatases are able to separate the 3-OH-sulphonate group from BA (desulphation of BA). However, little is known about overt sulphatase activity within the gut because of difficulties in manipulating obligate anaerobes. Thus, the combination of dysbiosis and BA dysmetabolism suggests a loss of microbiota function in IBD. Indirect mechanisms may also be involved. Gut bacteria could modulate intestinal sulpho-transferase activity (sulphation of BAs) through modulins that interfere locally with ileal epithelial cells or colonocyte signalling.51 In addition, gut microbiota could trigger overall BA biosynthesis—and 3-OH-sulphated BAs in particular—via the gut-liver axis through regulation of nuclear receptors, such as Farnesoid X Receptor (FXR) expressed in ileal epithelial cells.52
The anti-inflammatory properties of secondary BAs were first described 20 years ago based on the observation that chronic cholestasis is associated with immunosuppression. This led to the demonstration that BAs inhibit the secretion of TNF-α, IL-1β and IL-6 in macrophages.21 ,53 Recently, it has been established that this downregulation was mediated by the BA-specific membrane receptor TGR5. This G-protein-coupled receptor can reduce proinflammatory cytokine (IL-1 α, IL-1β, IL-6 and TNF- α) production induced by lipopolysaccharides in Kupffer cells and macrophages through NF-kB inhibition.19 TGR5 is mostly activated by secondary BAs, including LCA and DCA. Therefore, it is important to note that in humans, the strongest activator of the TGR5 anti-inflammatory receptor is produced by the gut microbiota. The anti-inflammatory effect of LCA has been described elsewhere.54 Interestingly, a TGR5 agonist can attenuate Trinitrobenzene Sulfonic acid (TNBS), which induces colitis in mice.55
We designed our in vitro experiments using a Caco-2 cell assay to model and understand whether the lower proportion of secondary BAs in faeces could influence inflammation pathways in colonic mucosa. These assays confirm that secondary BAs (LCA and DCA) can exert anti-inflammatory effects in human colonic epithelial cells independently of any cytotoxic effect of BAs on cells. Interestingly, this effect was lost after 3-OH-sulphation. Thus, one can hypothesise that the anti-inflammatory properties of secondary BAs on Caco-2 cells we report here could be TGR5 dependant.
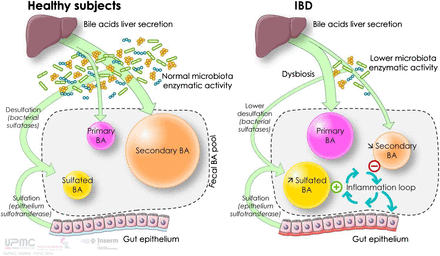
We propose the following physiopathological model, which implicates luminal BA dysmetabolism in the gut inflammation observed in IBD (figure 7). In HS, normal microbiota enzymatic activity leads to a physiological luminal BA pool that is mostly composed of secondary BAs and very low levels of primary and 3-OH-sulphated BAs. However, in IBD patients, impaired microbiota enzymatic activity leads to modification in the luminal BA pool composition, with increased sulphated BAs at the expense of secondary BAs. These changes contribute to the loss of the anti-inflammatory effects of secondary BAs on intestinal epithelial cells, while enhancing chronic inflammation.
Physiopathological model of luminal bile acid dysmetabolism in inflammatory bowel diseases. This figure is only reproduced in colour in the online version.
Dysbiosis in IBD is generally described as a qualitative and quantitative imbalance in the gut microbiota. This study establishes a credible link between perturbation of the gut microbiota composition and clinical outcomes. In light of this study, BA appears to be a new player in IBD, participating in the proinflammatory ‘vicious cycle’ between the gut microbiota and host.
Acknowledgments
The authors wish to thank MR Popoff, JP Carlier and M Manich of the Anaerobic Bacteria and Toxins Unit of the Pasteur Institute in Paris, France, Yves Chrétien of the Pierre and Marie Curie University in Paris, France, and Mrs Eugenia Hu for her assistance with the manuscript.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
HD, SR, PS and HS contributed equally
-
Contributors HD, SR, HS, PS wrote the paper. All the authors, except LB, JC, OC, RP, GT, PL, CW, JM performed the lab experiments and designs. LB, JC, OC, RP, GT, PL, JM gave critical revision of the manuscript. CW and DB performed statistical analysis.
-
Competing interests For the present work, the authors declare no conflict of interest. For the current year, Prof Seksik received consulting fees from Biocodex, Ferring pharmaceutical, MSD and Abbott. Prof Cosnes received consulting fees from Ferring pharmaceutical, MSD and Abbott. Prof Beaugerie received consulting fees from Biocodex, Ferring pharmaceutical, MSD and Abbott. The remaining authors disclose no conflict.
-
Ethics approval Local ethics committee.
-
Provenance and peer review Not commissioned; externally peer reviewed.