Article Text
Abstract
Background and aims Probiotics and their metabolic products, here called postbiotics, have been proposed as food supplements for a healthier intestinal homeostasis, but also as therapeutic aids in inflammatory bowel disease (IBD) with, however, very little clinical benefit. This may be due to the lack of reliable preclinical models for testing the efficacy of different strains.
Methods The activity of three probiotic strains of Lactobacillus (or a postbiotic) was analysed and compared with a pathogenic strain of Salmonella on a novel organ culture system of human healthy and IBD intestinal mucosa developed in our laboratory. The system maintains an apical to basolateral polarity during stimulation due to the presence of a glued cave cylinder. The cylinder is detached at the end of the experiment and the tissue is processed for histology and immunohistochemistry. Cytokines released from the basolateral side are analysed.
Results The model system provides several physiological characteristics typical of a mucosal microenvironment including the presence of an organised mucus layer and an apical to basolateral polarity. Polarised administration of bacteria is critical to control the ensuing immune response as it mimics the physiological entrance of bacteria. The authors show that probiotics are not always beneficial for the healthy host and can also be detrimental in inflamed IBD. This study shows that a potent postbiotic can protect against the inflammatory properties of invasive Salmonella on healthy tissue and also downregulate ongoing inflammatory processes in IBD tissue.
Conclusions Probiotics can have inflammatory activities in both healthy and IBD tissue. Valid preclinical data on proper model systems should therefore be obtained before specific probiotic strains enter the clinics, especially if administered during acute inflammatory responses. Postbiotics may be a safe alternative for the treatment of patients with IBD in the acute inflammatory phase.
- Immunology
- immunoregulation
- immunotherapy
- infectious disease
- intestinal bacteria
- Crohn's disease
- ulcerative colitis
- gut immunology
- gut inflammation
- immune response
- anti-bacterial mucosal immunity
- antigen presentation
- epithelial barrier
Statistics from Altmetric.com
- Immunology
- immunoregulation
- immunotherapy
- infectious disease
- intestinal bacteria
- Crohn's disease
- ulcerative colitis
- gut immunology
- gut inflammation
- immune response
- anti-bacterial mucosal immunity
- antigen presentation
- epithelial barrier
Significance of this study
What is already known about this subject?
The mechanisms of action of probiotics can vary between different species and within the same species between strains. These mechanisms can be complex and affect different cell types.
Probiotic action has been studied on isolated cells and cell lines, cell co-cultures and mouse models. These do not accurately represent the unique microenvironment of the intestine as they all lack important human-specific components like the mucus and the microbiota that are either absent or differ in the two species.
Results from these models are often contradictory between different cell lines/mouse strains and even between the same cell line or between mice handled by different researchers.
What are the new findings?
A new polarised organ culture system of human intestinal mucosa is described that retains characteristics of the complex intestinal microenvironment.
Even species of probiotics belonging to the same genus, in this case Lactobacillus, can behave very differently, even when applied on healthy mucosa.
A potent postbiotic can protect healthy epithelium from otherwise highly infectious agents such as Salmonella and can downregulate proinflammatory pathways active in IBD tissue.
How might it impact on clinical practice in the foreseeable future?
Postbiotics may be a safe alternative to probiotics during the acute phase of IBD.
The newly described polarised model system could be used to generate preclinical data for physiological testing of probiotics and postbiotics as well as other mucosal treatments on healthy or pathological tissues, thus allowing for safer clinical trials.
This model could be translated to other sites requiring polarised application of treatments such as the lungs, stomach and skin.
Background
The importance of a balanced nutrition and a healthy intestine for the well-being of humans has been known for many years.1 The intestinal mucosa, a vast surface of 200 m2, is the part of our body devoted to nutrient absorption and responsible for taking full advantage of what we ingest. This function is carried out with the help of resident commensal microorganisms (microbiota) that participate in the degradation of complex macromolecules.1 The microbiota co-exists harmoniously with its host, offering and acquiring many benefits.2 Hence, the gut mucosa is continuously challenged with microbial antigenic stimulation from normal intestinal flora and does not mount an immune reaction against it; however, it is still able to react to pathogenic bacteria.3 Dysregulation of the composition of the microbiota has recently been suggested as a possible risk factor leading to inflammatory bowel disease (IBD) in genetically susceptible individuals.4–6
IBD comprises two pathological conditions affecting the bowel—ulcerative colitis (UC) and Crohn's disease (CD). It is characterised by excessive inflammation in various parts of the intestinal wall, eventually leading to mucosal damage, ulcerations and fibrosis. Even though the pathogenesis of IBD is still unknown, current theories hypothesise that a dysregulated immune reaction against components of the normal luminal flora is a major driver to IBD-related mucosal inflammation. Indeed, several studies have documented an altered gut flora composition in individuals with CD and UC. For instance, a reduction in Faecalibacterium prausnitzii in the normal gut microbiota has been associated with recurrence of CD.7 Whether this represents a primary event responsible for IBD-related intestinal inflammation or a consequence of the IBD-related inflammatory milieu is still a matter of debate.8
Even if there is no definitive cure for IBD, currently available treatments for patients with UC and CD include aminosalicylates, steroids and immunosuppressive drugs such as thiopurines and anti-tumour necrosis factor (TNF) agents aimed at controlling mucosal inflammation. However, these treatments are beneficial only in a proportion of patients with IBD and surgery often remains the best therapeutic option.
Probiotics are living microorganisms which, when administered in adequate amounts, confer a health benefit on the host. The health benefits of probiotics are highly dependent on the bacterial strain9 10 and on the clinical setting in which they are used.11 Each probiotic species, and within each species each strain, may have distinct activities.9 10 Recent clinical studies have concluded that caution needs to be taken when administering probiotics in patients with acute inflammation. Indeed, treatment with a mixture of probiotics in patients with acute pancreatitis resulted in death in 16% of the patients in the probiotics group compared with 6% in the placebo group.12 Thus, before taking probiotics into the clinic, thorough preclinical studies have to be carried out.
We describe a human mucosa explant culture model in which an apical to basolateral polarity is preserved during stimulation with bacteria. With this model system we tested the activity of different probiotics on healthy and IBD tissues.
Methods
Tissue sampling, bacteria culture, histochemistry, gentamicin protection assays, cytokine secretion measurement and statistical analysis are shown in the online supplement.
Tissue culture and stimulation
Once in the laboratory, the clean mucosal layer was washed in Hank's Balanced Salt Solution buffer and cut with sterile scalpels into 1 cm2 pieces. The pieces were placed on sterile metal grids and the cylinder (cloning cylinder, various sizes, BellCo, Modena, Italy) was attached with surgical glue (Vetbond, 3M, Milan, Italy) under sterile conditions with a pair of forceps (figure 1). The culture medium was Dulbecco's Modified Eagle Medium supplemented with 15% freshly added fetal bovine serum, non-essential amino acids, sodium pyruvate, glutamine, epidermal growth factor (200 ng/ml, Peprotech, Milan, Italy) and Insulin-Transferrin-Selenium-X (10 μl/ml, Gibco, Monza, Italy). 1 ml of complete medium was dispensed in the centre well of the plate (Falcon, centre-well organ culture dish). Stimulation was performed with 4×107 colony forming units (CFU)/cylinder Salmonella typhimurium in 200 μl medium with or without Lactobacillus paracasei supernatant (SN) (5%). The same number of CFUs was used for inoculation of probiotics. Dilutions of bacterial cultures were plated onto LB or MRS agar plates to confirm the accuracy of the bacterial counts. As a control for the SN, (5%) unfermented bacterial medium (MRS) was added to the cylinder.
Application of a cylinder on the luminal face of an intestinal mucosal explant does not impact on tissue well-being. (A, B) Schematic representation and photographs of the technical setting for culture and apical stimulation. (C) Tissue cultured in the absence of the cylinder (left panel) and with the cylinder (right panel). Ellipse: cylinder contact point. Scale bars: 500 μm. (D) Left panel: Tissue stained with phalloidin-FITC in the interior of the cylinder (green) and 4′,6-diamidino-2-phenylindole (blue, for nuclear staining) once the cylinder was removed. Middle panel: The same piece of tissue inside the cylinder (magnification 4×). Right panel: Tissue processed with two cylinders of different size (magnification 1.5×). White lines represent cylinder borders.
After 2 h at 37°C in a 5% carbon dioxide incubator, the medium was removed from the inside of the cylinder and the tissue was transferred to the oxygen chamber. The chamber was filled with pressurised oxygen (VitalAire, Milan, Italy) and placed at 37°C for the remaining 22 h of culture.
Results
Technical set-up of the organ culture model to achieve polarised stimulation
The interaction of bacteria with intestinal mucosa is complex and comprises several key events including attachment/degradation of mucus, competition with the commensal microbiota and resistance to antimicrobial peptides produced by the epithelial barrier.13 To meet these requirements, we developed a new model of the intestinal mucosa which resembles as closely as possible the in vivo situation by setting up a polarised organ culture system. Healthy mucosal tissue (explant) was obtained as described in the online supplement. During the transfer the tissue was preserved in buffer with or without bacteriostatic antibiotics in order to conserve the microbiota. However, in both cases the superficial part of the mucus layer was washed off, but the microbiota adhering to the remaining mucus proliferated during the overnight culture and colonised the newly produced mucus (as attested by fluorescence in situ hybridisation, see figure 1 in online supplement). In the absence of antibiotics during transfer, the commensals hyperproliferated and contaminated the basolateral culture medium (not shown). We therefore decided to transfer the tissue in buffer containing bacteriostatic antibiotics. Once in our laboratory, the mucosa was cut into pieces of about 1 cm2 and placed on metal grids with the basolateral side facing downwards (figure 1A,B). The whole ensemble was then brought onto a centre-well tissue culture plate containing 1 ml of medium. At this stage only the basolateral side of the tissue was in contact with the medium (figure 1B(i)). Following previously published protocols, we compared culture in conventional incubators with culture in a 99% oxygen-saturated atmosphere and observed that both healthy and IBD tissues were only successfully cultured in the presence of oxygen (see figure 2 in online supplement).
The ability of luminal bacteria to invade the epithelium and to enter the intestinal lamina propria (LP)—a characteristic associated with pathogenic bacteria—dictates the outcome of the elicited immune response. For instance, the engagement of Toll-like receptor (TLR)-9 by bacteria-derived unmethylated CpG sequences on the apical surface of epithelial cells induces partial activation of nuclear factor-κB (NF-κB) without stimulating the release of proinflammatory cytokines.14 In contrast, basolateral engagement of TLR9 leads to a full inflammatory response which can be inhibited by preincubating the epithelium with TLR9 ligands applied on the apical side.15 This suggests that ‘sensing’ of pathogens depends on their capacity to deliver microbial components either intracellularly or to the basolateral compartment of the epithelium for engagement of pathogen recognition receptors. Thus, to mimic the ‘natural’ condition, we sought a way to maintain the apical to basolateral polarity of the tissue during the stimulation. To this end, a cave cylinder was attached to the apical side of the mucosa with surgical glue (figure 1A,B). We then evaluated the potential detrimental effects of these components (cylinder and glue) on the explants. The cylinder did not greatly affect the three-dimensional structure of the tissue (figure 1C), causing only minimal rupture effects after careful removal for immunohistochemical evaluation. Measurement of lactate dehydrogenase release showed that the presence of the cylinder or the glue did not cause the tissue to undergo necrosis (not shown).
Finally, we evaluated the tightness of the cylinder by incubating a solution containing FITC-conjugated phalloidin inside the cylinder glued on the tissue. The cylinder was then taken off and the whole explant stained with 4′,6-diamidino-2-phenylindole and visualised under a fluorescent stereoscope. The phalloidin was confined within the cylinder, confirming the absence of leakage (figure 1D). To show the flexibility of our system, two cylinders of different diameters were attached to the explant, one inside the other, and cultured with FITC- or TRITC-conjugated phalloidin. The two different compartments were kept completely separate throughout the culture (figure 1D, right panel).
Bacterial stimulation and advantage of the cylinder
We reasoned that oxygen might affect the viability of both the existing microbiota and the bacteria used for stimulation, so tissues were incubated for 2 h in a conventional incubator—that is, in the absence of additional oxygen—in the presence or absence of S typhimurium (4×107 CFU in 200 μl medium) and in the absence of antibiotics. The bacteria were then washed off and the medium was substituted with one containing antibiotics and transferred to the oxygen chamber. However, we found that the presence of the medium in the cylinder damaged the tissue even when the sample was not treated with bacteria (figure 3 in online supplement: compare panel B inside with panel A outside the cylinder). Within the cylinder the tissue showed glandular destruction with detachment from the basal membrane, extensive apoptosis, capillary permeation and haemorrhagic suffusion (figure 3B in online supplement). This was probably because the culture medium prevented the exchange of oxygen as the tissue outside the cylinder that was exposed to oxygen was in perfect condition (figure 3A in online supplement). We therefore determined whether transfer to the oxygen chamber after the first 2 h of stimulation in a conventional incubator in the absence of medium inside the cylinder would preserve tissue viability. This would allow bacterial stimulation to be performed in the best bacterial culture conditions and preservation of tissue structure in the oxygen chamber. Tissue stimulated under these conditions (figure 4 in online supplement) was as healthy as the tissue left for 24 h in the oxygen chamber and not much different from the tissue before treatment (figure 3C in online supplement). Moreover, as shown in figure 4 in the online supplement, these conditions preserved the structure of the tissue and also the mucus layer throughout the culture. We also monitored tissue architecture over time and found that areas of tissue degeneration could be observed after 36 h (see figure 4 in online supplement).
We then confirmed that the cylinder was actually confining the infection by S typhimurium to the apical face. To avoid exposing larger surfaces when treating tissues without the cylinder, we cut the mucosa into pieces the size of the cylinder (figure 2A). The pieces were then stimulated with Salmonella (4×107 CFU/explant) contained or not contained within the glued cylinder. We expected to observe increased production of the inflammatory cytokine TNFα (a cytokine required for Salmonella clearance16) in samples where Salmonella was layered on the tissue without being confined in the cylinder and hence allowed to enter also via the broken edges (ie, through a non-physiological route; figure 2A). This would allow Salmonella to access the basolateral side of the epithelium and LP leucocytes for enhanced stimulation of TNFα secretion (figure 2A). Indeed, TNFα was produced in much greater quantities by explants stimulated with Salmonella in the absence of the cylinder, regardless of the pathological conditions (figure 2B). Immunofluorescence to assess Salmonella localisation in the tissue after incubation for 2 h (and immediate subsequent fixation) with or without the cylinder confirmed the penetration of Salmonella from the non-apical side and direct contact with LP components in the absence of the cylinder whereas, where the cylinder was present, Salmonella had only penetrated the mucus layer apically after 2 h of stimulation (figure 2C).
The cylinder confines the Salmonella-induced inflammatory response. (A) Schematic representation and photographs of tissue treated with Salmonella within the cylinder (left) or without (right). Without the cylinder, Salmonella can penetrate the tissue via its broken edges. (B) Tumour necrosis factor α (TNFα) secretion of two healthy samples (H1 and H2, left) and two ulcerative colitis samples (UC1 and UC2, right) after stimulation with Salmonella in the presence or absence of the cylinder. Error bars represent SEM of three separate measurements on three parallel experiments measured by ELISA,**p<0.01; ***p<0.001. (C) Immunofluorescence of tissues treated with Salmonella with or without the cylinder as in (A) for 2 h. Anti-Salmonella (red) and nuclei (blue, 4′,6-diamidino-2-phenylindole) stainings are shown. In the absence of the cylinder, Salmonella penetrates from the broken edges of the tissue and comes into direct contact with the components of the lamina propria. Scale bars: 200 μm.
Probiotics have diverse activity on healthy and IBD tissues
The use of probiotics in the clinic to ameliorate the symptoms of IBD has been proposed but with limited clinical outcomes. We used our newly set-up method to evaluate the response of healthy or IBD mucosa to Lactobacillus paracasei B21060, L rhamnosus GG (LGG) or L plantarum NCIMB8826. These probiotic strains were chosen based on a previous study in our laboratory on a complex co-culture model of epithelial cells and dendritic cells.17 As expected, we did not detect significant changes in the well-being of healthy tissues after inoculation of L paracasei or LGG (figure 3), and the cytokine secretion profile remained similar between stimulated and negative control samples (see figure 5 in online supplement). Surprisingly, however, stimulation with L plantarum resulted in a clear deterioration in the tissue at the end of the culture (figure 3B, right panel), with homing towards the epithelial layer of immune cells that comprise the lymphoid aggregates usually found dispersed throughout the colon below the LP (figure 3C). This reaction was observed on all three samples stimulated with L plantarum on which this aggregate was found on the analysed sections, while in one further sample we did not detect the lymphoid aggregate. Consistently, L plantarum inoculation resulted in a slight but significant upregulation of CCL4, one of the key molecules for immune cell homing to the site of inflammation.18 Interleukin (IL)-1β was also significantly increased after L plantarum but not after L paracasei or LGG inoculation (see figure 5 in online supplement). In contrast, when the tissues were treated with LGG and L plantarum in the absence of the cylinder, thus allowing the bacteria also to come into contact with the broken edges of the tissue, both strains induced an increased inflammatory response (see figure 6 in online supplement). This further confirms the necessity of the cylinder to confine the stimulation to the apical side of the mucosa for proper evaluation of the probiotic activity of the different strains tested. Interestingly, L paracasei did not exhibit proinflammatory properties even when allowed to penetrate basolaterally (not shown).
Not all strains previously described as probiotics are innocuous. (A) Negative control samples, tissue upon arrival (left) and tissue cultured for 24 h (right). (B, C) Tissues were incubated with either Lactobacillus paracasei, L rhamnosus GG or L plantarum for 2 h and then cultured for a further 22 h in 99% oxygen atmosphere. L plantarum causes tissue damage (B) and translocation of lymphoid aggregates to the apical surface (C, compare left and middle panels with right panel). Scale bars: 200 μm.
Contrary to these results, probiotic inoculation on IBD mucosa (4 patients with CD (2 colon and 2 ileum) and 2 patients with UC) completely altered the structure of the tissue, regardless of the strain (figure 4). This suggests that, when a tissue is already inflamed and there is probably increased permeability and translocation of otherwise non-invasive bacteria, bacteria that would normally be harmless may worsen inflammation, suggesting that caution should be taken when using probiotics in patients in the acute phase of the disease.
Challenge with whole probiotic bacteria on inflammatory bowel disease tissue leads to extensive tissue degeneration. (A) Negative control samples, tissue upon arrival (left) and tissue cultured for 24 h (right). (B) Crohn's disease (CD) tissue was stimulated for 2 h with the different probiotic strains and then cultured for a further 22 h in an oxygen chamber. Haematoxylin/eosin staining is shown. Scale bars: 200 μm. Pictures are representative of four CD samples (two colon, two ileum).
L paracasei supernatant inhibits the inflammatory potential of Salmonella
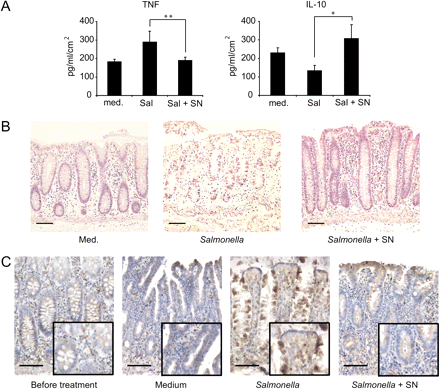
As mentioned above, whole bacteria can have a detrimental effect on pathological tissue. We recently showed that L paracasei is capable of inhibiting the inflammatory potential of dendritic cells and epithelial cells in complex co-culture systems in response to S typhimurium,17 and that this capacity is not associated with the bacteria but with a soluble mediator (postbiotic) released in the culture medium.17 This characteristic was unique among the three tested strains (unpublished results). A postbiotic could therefore be a safer alternative to the use of whole bacteria. We first confirmed the anti-inflammatory activity of the SN in our model system after Salmonella infection of healthy tissue. We measured the secretion of TNF and IL-10 (a proinflammatory and an anti-inflammatory cytokine, respectively) from the basolateral side of the sample. The tissue was processed as described above, and pieces were stimulated with either Salmonella (4×107 CFU/explant), Salmonella with 5% L paracasei SN, 5% SN alone or simply medium for 1–2 h in a humidified 5% CO2 incubator. Stimulation and immune response evaluation were carried out as described above. We observed an increase in the secretion of TNF after stimulation with Salmonella (figure 5A). However, when SN was added together with Salmonella in the cylinder, this effect was abrogated (figure 5A). SN alone had little or no effect on TNF production by the tissues (see figure 7 in online supplement). However, IL-10 production, which was reduced after Salmonella infection, was not diminished in samples treated with Salmonella plus SN or SN alone (figure 5A and figure 7 in online supplement), indicating that the SN has a dramatic effect on the inflammatory potential of Salmonella without affecting the anti-inflammatory response of the tissue. Parallelling the production of inflammatory cytokines, histological analyses of the tissues showed that we could mimic a classic Salmonella infection with ulceration of the epithelium, crypt necrosis, glandular destruction and superficial atrophy (figure 5B middle panel). In addition, we observed translocation of lymphoid aggregates towards the epithelium in response to Salmonella (reminiscent of that observed with L plantarum), and this translocation was drastically reduced when Salmonella was co-incubated with SN (figure 8 in online supplement). TNF production is a consequence of NF-κB activation, so we evaluated the translocation of p65 to the nucleus after Salmonella infection. As opposed to untreated and control samples, in the sample treated with Salmonella, p65 translocated to the nucleus in many of the LP components and even in epithelial cells (figure 5C, third panel). However, samples stimulated with Salmonella in the presence of SN only showed a slight NF-κB p65 upregulation on the uppermost layer of the mucosa whereas the rest of the tissue resembled control samples (figure 5C, right panel).
Lactobacillus paracasei culture supernatant (SN) counteracts Salmonella-induced inflammation. (A) Tumour necrosis factor (TNF) secretion (left) and interleukin 10 (IL-10) secretion (right) after 2 h of stimulation with Salmonella or Salmonella + SN and a further 22 h culture in oxygen; medium was used as a control for basal secretion measurement. Cytokines were measured by cytometric bead array; error bars represent SEM of eight samples and statistical significance was determined by the Wilcoxon test. *p<0.05; **p<0.01. (B) Representative tissue histologies of samples stimulated as described with either medium (left), Salmonella (middle) or Salmonella in the presence of 5% SN (right). (C) Nuclear factor-κB activation as evaluated by p65 localisation in the nucleus (dark brown) after Salmonella with or without SN stimulation as in (B). Scale bars: 200 μm, insets: 2×.
L paracasei supernatant conditions the epithelium against Salmonella invasion
We then evaluated the mechanism responsible for the reduced inflammatory response elicited by Salmonella in the presence of SN. We first assessed whether the SN affected Salmonella proliferation. We cultured Salmonella with or without SN and plated culture dilutions 30 min and 3 h later. The difference between the two cultures was not significant (see figure 9A in online supplement). We then evaluated whether the postbiotic affected the capacity of Salmonella to invade the tissue. When inoculated alone, Salmonella successfully penetrated the epithelium and diffused throughout the explant (figure 6A). However, in the presence of SN, Salmonella appeared to be unable to penetrate the deepest layers of the LP and was mostly retained in the epithelium. To quantify the capacity of Salmonella to invade the tissue, we performed a gentamicin protection assay on the tissue after 1.5 h of Salmonella stimulation with or without SN. Tissues were incubated for 1.5 h with medium plus antibiotics (gentamicin) to kill bacteria that had not entered the tissue. They were then lysed and Salmonella CFUs were quantified. We observed that the number of internalised bacteria was significantly lower (around 30%) in the presence of SN than when it was absent (see figure 9B in online supplement). This difference could be due to a direct action of SN on the bacteria or on the tissue. To address this issue we preincubated Salmonella or tissue with or without SN for 1 h. The bacteria and tissue were then extensively washed before being brought in contact for an additional hour. As shown in figure 6B, only when the tissue was conditioned with SN was Salmonella less invasive. Moreover, preconditioning of the tissue further affected the invasiveness of Salmonella as the number of recovered CFUs was much lower than when SN was incubated simultaneously with the Salmonella (compare figure 9B in the online supplement with figure 6B). This indicates that SN does not act on the Salmonella but directly on the tissue and does not need to be present at the time of infection.
Lactobacillus paracasei culture supernatant (SN) acts on the epithelium to protect against Salmonella invasion. (A) Anti-Salmonella staining of tissues stimulated with Salmonella in the absence (middle panel) or presence (right-panel) of SN. Untreated tissue fixed upon arrival (left panel) was used as a negative control. Scale bars: 500 μm. (B) Tissue, Salmonella or both were conditioned or not for 1 h with SN and then extensively washed before being brought in contact for 1 h. Tissues were then treated with gentamicin for a further hour to kill extramucosal Salmonella, lysed and plated to evaluate colony forming units (CFUs). Bars show total CFUs of internalised Salmonella. Error bars represent SEM of four experiments; ***p<0.001 (ANOVA).
L paracasei supernatant alone is sufficient to ameliorate inflammation in IBD tissues
We then evaluated the effect of SN in pathological tissues. In general, patients with CD have a basal increase in TNF expression in the tissue19 and anti-TNF therapy is one of the options for the treatment of CD.20 Incubation of SN on colon or ileal tissues from patients with IBD resulted in a significant reduction in TNF production in most of the patients (figure 7A). Moreover, we also observed a significant reduction in most of the cytokines and chemokines involved in the pathology of IBD including CCL4, CCL2, interferon γ and IL23p40 on tissues incubated with SN (figure 7A). By contrast, IL-10 secretion was not significantly altered after SN treatment, suggesting that the postbiotic did not interfere with the capacity of the tissue to counteract an inflammatory response. IL-17A was not significantly altered, nor were the Th2 response-related cytokines (IL-5, IL-13 and IL-4; not shown). Immunohistochemistry data for NF-κB activation were in agreement with the aforementioned results, with nuclear translocation of p65 significantly reduced on ileal CD tissues after treatment with SN (figure 7B,C, compare middle to right panel, healthy tissue was used as a negative control for NF-κB activation). As the replacement of these populations with incoming ones is not possible in this model, this would indicate that the SN is capable of downregulating the proinflammatory activity of existing leucocytes. Thus L paracasei SN seems to be a potent and safe anti-inflammatory agent with potential therapeutic use in patients with IBD.
Lactobacillus paracasei culture supernatant (SN) is capable of significantly downregulating basal secretion of proinflammatory cytokines and chemokines and greatly reduces nuclear factor-κB (NF-κB) activation on inflammatory bowel disease tissue. (A) Tissues were incubated with medium (C-) or 5% SN as described in the text. Cytokines were measured in the basolateral culture medium by cytometric bead array; *p<0.05, **p<0.01. (B) Immunohistrochemistry for NF-κB (p65, brown) after incubation of a representative Crohn's disease (CD) tissue sample with or without SN as described in the text. Healthy tissue was used as a negative control for nuclear p65. Scale bars: 100 μm. (C) Quantification of results shown in (B). 2.5mm2 areas were counted on three different samples; **p<0.01 (Wilcoxon test).
Discussion
Understanding how probiotics, prebiotics or postbiotics work in preclinical models that resemble the human situation can allow a ‘rational’ choice of the different strains or compounds for clinical and/or commercial use according to the pathological condition to which they are targeted. Indeed, we recently demonstrated that different Lactobacilli species can be classified as either immunogenic or tolerogenic.17 Testing of different probiotics has often been performed on isolated immune cells or peripheral blood mononuclear cells.21 However, particularly in the gut, the interaction of bacteria with the mucosa is an unusually complex event requiring attachment or degradation of mucus, competition with the microbiota and resistance to antimicrobial peptides.13 In addition, the function of immune cells is influenced by the local microenvironment and this is required to preserve intestinal homeostasis.4–22 Indeed, we have shown that signals from intestinal epithelial cells can drive the differentiation of non-inflammatory dendritic cells that differ substantially from their blood-derived counterparts.23–25 On the other hand, under inflammatory conditions, new populations of immune cells are recruited at inflamed sites, such as a new subset of CD205+CD209+ LP dendritic cells that are enriched in the gut of patients with CD and share markers with macrophages like CD14 and CD68.26 The development of a model system that resembles the human situation is therefore of great value for testing the action of anti-inflammatory agents on both healthy and diseased tissues.
In this report we show that incubation of whole tissue with bacteria can lead to unreliable results as the bacteria can penetrate the tissue via a non-physiological route (eg, from its broken edges) even if they are not invasive and enter into direct contact with components of the LP. We therefore generated a polarised organ culture system that also preserves the barrier properties of the mucus layer and its own microbiota. We achieved this by gluing a cylinder onto the apical side of the tissue, physically isolating the area of stimulus application. This allows the targeting of only the area that is exposed to the intestinal lumen, thus mimicking the in vivo situation as closely as possible. This methodology was also successfully used to analyse more fragile tissues such as those isolated from patients with IBD. To our knowledge, this is the first demonstration of the possibility of culturing a tissue in a polarised fashion for up to at least 24 h in optimal conditions.
We compared the activity of probiotics on tissue from the same patient and from the same area of the intestine, thus allowing for comparison with the basal cytokine secretion level and highly reproducible results. Bacterial stimulation was carried out in a conventional incubator for best bacterial growth conditions; however, the presence of oxygen was not killing the bacteria. Increased oxygen pressure is found at the interface between the epithelium and the lumen,27 indicating that hyperoxygenation may represent a physiological requirement. We also mimicked Salmonella infection in terms of pathology of the tissue and immune cytokine response. We observed an evident translocation of lymphoid aggregates and ulceration of the epithelium, with extensive crypt necrosis, glandular destruction and superficial atrophy. With this system we demonstrated an anti-inflammatory effect of a probiotic SN on Salmonella-induced inflammation. We showed that, although Salmonella alone induced TNF production, its co-incubation with the postbiotic resulted in the abrogation of TNF release without affecting IL-10 production. This was dependent on a direct effect of the SN on the tissue and not on Salmonella, and was enhanced when the tissue was preconditioned. We showed that the SN also exerts its anti-inflammatory potential on IBD tissues. We also unexpectedly observed that not all probiotics are harmless even on healthy tissues, and some strains such as L plantarum NCIMB8826 can induce a local inflammatory response that resembles the response induced by Salmonella. By contrast, all tested probiotic strains, including L paracasei, which we earlier showed to be protective in a mouse model of experimental colitis,17 are detrimental on inflamed IBD tissues. This is probably due to the increased intestinal permeability in IBD tissues28 29 which we speculate could lead to increased bacterial translocation through the epithelial monolayer and activation of underlying infiltrating cells.
Together, our data indicate that probiotics would be more adequately used in patients in remission and not during the acute phase of the disease. However, this cannot be generalised to all strains and case-by-case preclinical studies on valid models are required before entering the clinics. The use of a postbiotic like the one described here could be an effective and safe alternative for the treatment of acute IBD.
Acknowledgments
We thank Erika Mileti for excellent technical support and Dr Antonio Di Sabatino for providing the oxygen chambers.
References
Supplementary materials
Gut education editor Mairi McLean talks to Maria Rescigno in the Gut podcast
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
- Download Supplementary Data (PDF) - Manuscript file of format pdf
Footnotes
Funding This work was supported by grants from the 7th EU framework program (IBDase, ERC: Dendroworld) and Fondazione Cariplo to MR and support by a Marie Curie international training mobility network (Cross-Talk, grant agreement No: 21553-2) to KT.
Competing interests None.
Ethics approval Only material that was not required for diagnosis was used and all patients signed an informed consent approved by the IEO Institutional Review Board which allows researchers to use exceeding material for research purposes.
Provenance and peer review Not commissioned; externally peer reviewed.